Macromolecular Damage Ages Us Prematurely – Autophagy Helps
Summary: Macromolecular damage contributes to the chronic diseases of aging by causing DNA damage, inflammaging, stem cell decline, increased cellular stress and cell death. Hopefully, geroscientists may have found a way to repair the damage using compounds like rapamycin that induce autophagy. This article first appeared on the LongevityFacts.com website. Author: Brady Hartman.
Macromolecular Damage Ages Us Prematurely
As we grow older, the decades of damage to our cells and tissues called macromolecular damage, cause the chronic diseases of aging and lead to our gradual decline.
To reverse aging, we need a way to clear out decades of macromolecular damage in viable, yet aged cells. The best way to accomplish this is by prodding the cell’s natural housekeeping process known as autophagy. Unfortunately, our natural autophagy processes decline as we age. Scientists have found that the drug rapamycin stimulates autophagy but other tricks also boost our cellular housekeeping.
When a cell has become too damaged, the best course of action for the body is to eliminate this cell completely. For example, researchers have developed, a new breed of drug called senolytics that reverses aging in some tissues of mice by removing crippled cells. Researchers are planning to test these drugs in humans.
However, even if senolytic compounds are wildly successful – which we all hope for – senolytic treatments will leave our tissues full of viable yet damaged cells. These tissues, chock full of macromolecular damage, will continue to promote disease and dysfunction. To reverse aging, we need to clean out this damage as well.
Does Macromolecular Damage Cause Aging?
Leading geroscientists such as Carlos Lopez-Otin and others agree that the deterioration of the building blocks of our cells, called macromolecular damage, is a significant hallmark of aging. There is little disagreement that macromolecular damage accompanies aging. The dispute is over whether this damage is the root cause of aging or just a byproduct of the aging process itself.
Two respected authors teamed up to study the role of macromolecular damage in aging; Professor Arlan Richardson, Ph.D., from the Department of Geriatric Medicine and University of Oklahoma Health Science Center; and Eric Schadt, Ph.D., with the Department of Genetics and Genomic Sciences, Mount Sinai School of Medicine. The authors published a paper with their findings stating,
Several decades of research have shown that macromolecular damage increases with age and that damage to protein, DNA, lipids, and other macromolecular components appears to be important factors in specific age-related diseases.
As we age, damaged cellular components accumulate in and around our cells. These damaged building blocks of our cells, called macromolecules, include DNA, lipids, proteins, and carbohydrates.
Richardson and Schadt suspect that macromolecular damage is a root cause of aging. For evidence, the authors quote studies which show that calorie restriction increases lifespan, saying
The strongest evidence that macromolecular damage is a causative factor in aging comes from studies using manipulations that increase life span.
Richardson and Schadt are cautious about offering a final verdict, stating
However, it is currently unclear whether damage to macromolecules plays a role in the actual processes of aging. In other words, is macromolecular damage driven by aging or is it that damage to a key molecular component directly causes aging?
Macromolecular Damage as a Driver of Disease
While geroscientists have yet to come up with the proximal cause of aging, it is apparent to scientists, physicians, and the average person that damage to cells and tissues causes disease. For example, damage to beta cells cause type 1 diabetes; damaged arteries, combined with high cholesterol cause atherosclerosis; and damaged heart cells contribute to heart disease. Macromolecular damage is a significant cause of illness.
Various scientists offer evidence that macromolecular damage contributes to cellular senescence, stem cell decline, progenitor cell dysfunction, and inflammaging, a chronic low-grade inflammation that usually accompanies old age. In turn, researchers say that these conditions significantly contribute to the major chronic diseases of aging, such as diabetes, Alzheimer’s and heart disease.
Bottom Line: Macromolecular damage significantly contributes to chronic diseases. However, researchers are unsure if this damage causes the aging process itself.
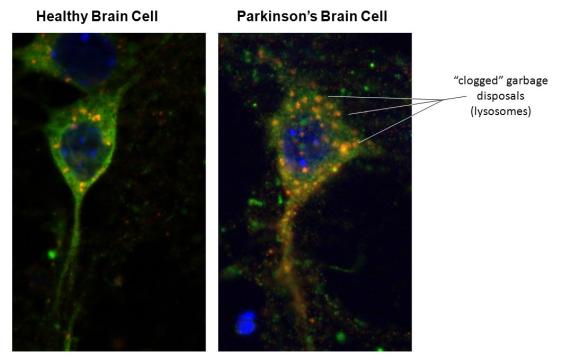
Macromolecular Damage to Proteins
By and large, proteins are the most significant class of macromolecular damage. Proteins make up the building blocks of our cells and tissues and can fall victim to all sorts of hazards. Dysfunctional proteins include those that are misfolded, oxidized, abnormally glycated, cross-linked, and aggregated. These damaged proteins do not just take up space. As we age our protein quality control and autophagy processes degrade and our cells and tissues fill up with these junk proteins which promote inflammaging, cellular senescence, and cell death.
Even the cautious Richardson and Schadt suspect damaged proteins as causing disease and aging, and as they say in their study,
How could damage to protein lead to aging? Because proteins play a critical role in catalyzing reactions that are critical to a cell…
Look into a microscope, and you can find these dysfunctional proteins at the scene of the crime, in the tissues of affected organs in age-related chronic diseases. If you don’t have a microscope handy, look at the image above, and as an example, you can see how a decline in autophagy up due to clogged lysosomes lets macromolecular damage build-up in Parkinson’s Disease. Aggregated proteins both accompany and worsen many diseases, such as Alzheimer’s, Parkinson’s disease, insulin-dependent diabetes, and dilated cardiomyopathy.
Macromolecular Damage Weakens Stem Cells
Our cells become damaged all the time, and fortunately, our bodies are equipped with stem cells to repair the damage. To do the job, our tissues are full of progenitor cells – the early descendants of stem cells. Progenitor cells have a limited capacity for self-renewal and play a vital role in replacing and repairing damaged tissue. Like the shmoos of the cellular world, progenitors can differentiate into specialized cells to make new tissue.
Unfortunately, as the decades roll on, our stem cells decline due to years of damage. This happens as the build-up of damaged macromolecules stimulates cellular stress responses in the progenitor cells. In turn, this stress inhibits them from differentiating into specialized cells. No differentiation, no new tissue.
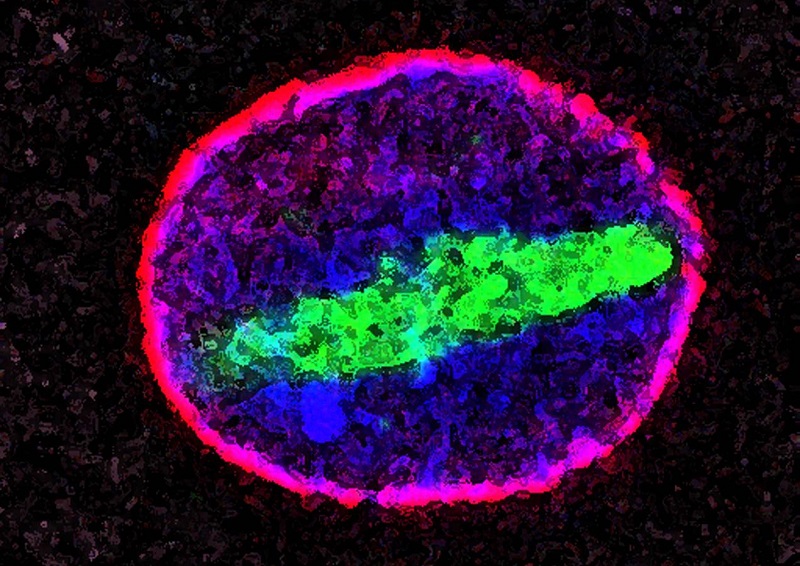
Macromolecular DamAGE Causes Inflammation
While there is plenty of types of macromolecular damage, for an example of how they lead to chronic diseases, consider the case of glycated proteins.
Proteins frequently get tangled up with a sweet young thing called sugar, inappropriately binding with them to form advanced glycation end-products. Glycation occurs when a sugar molecule chemically attaches to something it ought not to, such as a protein, a lipid (fat) or a nucleic acid (part of DNA). When sugar latches on to one of these macromolecules, it forms an advanced glycation end-product. It’s a bad marriage that leads to all kinds of trouble.
Our Declining Defenses Against the AGEs
Our defenses against the formation of advanced glycation end-products weaken as we age. Advanced glycation end-products are bad news because they stoke inflammaging and have been implicated in a slew of chronic diseases. Our bodies are finely tuned to advanced glycation end-products and even have receptors for them, called Receptors for Advanced Glycation End-Products (RAGEs) that set off pro-inflammatory cascades. AGEs are but one of the causes of the low-grade chronic inflammation that accompanies aging called inflammaging. In turn, inflammaging contributes to host of dysfunction and chronic diseases.
AGEs in Clinical Trials
Experimental evidence supports the idea that AGEs promote inflammaging. For example, a group of Italian scientists wanted to see how much damage is caused by dietary AGEs and set up an experiment to find out.
The researchers put a group of prediabetics on either a low AGE or standard diet. After about six months, the researchers found that, compared to the control group, the group on the low AGE diet low had improved cholesterol levels and reduced C-reactive protein (CRP), a marker of inflammation. They published their results in 2016 in the Journal of Clinical Lipidology.
Bottom Line: The Italian experiment showed that a diet low in AGEs resulted in lower levels of inflammation and vice-versa.
Diseases Caused by Advanced Glycation End-Products
One danger of advanced glycation end-products is that they can clog the tiny blood vessels throughout the body – called the microvascular system – especially those in the brain, kidneys, eyes, and heart. This clogging of the microvascular system may contribute to the risk of various complications of diabetes.
Physicians have long known that high blood sugar leads to all kinds of problems in the body, and this may be due in part to high levels of advanced glycation end-products. High blood sugar leads to increased inflammation and more AGEs, two known sources of disease and damage. In diabetics, uncontrolled blood sugar leads to higher risks of all kinds of complications, including heart disease, stroke, kidney disease, amputations, and blindness.
High levels of AGEs can be found in many healthy older adults as well as in those with chronic diseases, so scientists are unsure of how strong a role they play in human health. Scientists have not conclusively fingered AGEs as the culprit behind these diseases, but they are suspect.
Macromolecular Damage Causes Cardiovascular Disease
For example of how macromolecular damage leads to disease, consider the concept that AGEs promote heart disease. Geroscientists strongly suspect that AGEs work in three ways to stoke the pathology of atherosclerosis. In turn, atherosclerosis, also called hardening of the arteries, leads to heart disease. While many things lead up to a heart attack, the concept that AGEs, a form of macromolecular damage, contribute to heart disease goes as follows
Strike One: AGEs can cause crosslinks to form in the collagen in our artery walls, stiffening them up and making it easier for cholesterol to stick to the walls of our artery.
Strike Two: Cholesterol can also become glycated, making it more likely to become oxidized. Oxidized LDL cholesterol stokes the fires of atherosclerosis.
Strike Three: AGEs bind to the RAGE receptors and cause oxidative stress as well as stimulate inflammatory pathways in the lining of our arteries known as vascular endothelial cells. This inflammation attracts immune cells until a crowd forms.
Three Strikes and Out: Our veins are the last place we want a crowd to form. If too many immune cells gather, a blood clot forms, which can break off and lodge in an artery feeding the heart or brain, leading to heart attack or stroke.
Macromolecular Damage to Lipids
Lipid damage is another form of macromolecular damage and, unfortunately, our lipids also become damaged as we age. Lipids are fats, and while you think we should avoid fats, our bodies need them for many essential functions.
Our bodies typically store fat in adipocytes, also called fat cells. Adipocytes have three essential jobs. First of all, they store energy in the form of fat and respond to insulin to meet the immediate energy needs. Secondly, they secrete hormones that our bodies need. Lastly, adipocytes keep toxic fat out of the areas in which it does not belong. Some fat is toxic to our tissues, and fat cells are much better equipped for storing this toxic waste.
More On the Topic of Ectopic Fat
Ectopic fat is a type of fat that collects in abnormal locations in the body and adversely affects our health. Ectopic fat accumulates in the liver, bone marrow, pancreas, muscle and around some of our organs, where it is called visceral fat. The age-related frailty common to many seniors, called sarcopenia is linked to ectopic fat infiltration of muscle.
Scientists do not precisely know why fat gets stored in the wrong places. Some blame it on the decreasing capability of our adipose tissue (fat cells) to store fats efficiently. Geroscientists believe this due to the fat cell progenitors failing to differentiate into fully functioning fat cells. Our progenitor cells have also taking am age-induced damage hit, and are less efficient.
Ectopic Fat Promotes Disease
Besides being ugly to look at, ectopic fats are unhealthy because they contain reactive molecules, cytotoxic fatty acids, and ceramides. While our adipose (belly fat) tissue can store these toxic wastes without harm, the other cells in our body are poorly equipped to protect themselves against these toxins. Too many ectopic fats can lead to a condition known as lipo-toxicity, which in turn contributes to metabolic dysfunction and inflammaging. The problem is made worse in people who overeat.
However, don’t blame your overactive appetite too much, because autophagy also declines with aging and this decline contributes to the accumulation of intracellular cytotoxic fats.
Bottom Line: Lipid damage is a form of macromolecular damage. In general, as we age, our defenses against lipo-toxicity decline at all levels, and this amplifies the adverse health effects of the build-up of ectopic fats.
Are Telomeres Part of Macromolecular Damage?
Our DNA has protective caps called telomeres which start out long and naturally shorten as a cell divides. However, Richardson and Schadt make the point that telomeres are macromolecules as well, and are subject to all kinds of damage. The authors point out that abnormally short telomeres lead to disease and accelerated aging. The concept that telomere damage leads to aging remained an unproven theory until Dr. John Cooke recently showed that lengthening telomeres reversed aging in cells in culture.
Related Article: Learn more about the idea of lengthening telomeres to rejuvenate the body in this report.
Crippled DNA is Part of Macromolecular Damage
DNA is another macromolecule that becomes damaged as we grow older. DNA damage happens all the time. Our body’s DNA repair mechanisms usually fix the damage, but errors slip through and accumulate as we age. The deterioration of our DNA repair machinery contributes to aging, as these errors stack up.
Even the cautious Richardson and Schadt strongly suspect damaged DNA as causing disease and aging. The authors reasonably sure about nuclear DNA damage giving rise to cellular senescence and cell death, however, they are less sure about the damage to mitochondrial DNA as a cause of aging, as they say in their study,
How could damage to DNA cause aging? First, persistent DNA damage could accumulate with age and interfere with transcription.” adding further on “Second, DNA damage can give rise to cellular responses, such as cellular senescence and apoptosis.” concluding with “Therefore, it seems likely that mutations in nuclear DNA are an important causal factor in aging. The same may be true for mutations in mitochondrial DNA; in particular, large deletions may drive accelerated aging in mouse models.”
Repairing DNA Damage
However, there is hope. As a recently published report on DNA damage shows, researchers have made headway in understanding how to stimulate DNA repair. Furthermore, the NAD-boosting compound nicotinamide mononucleotide (NMN) seems to boost natural DNA repair mechanisms in lab animals.
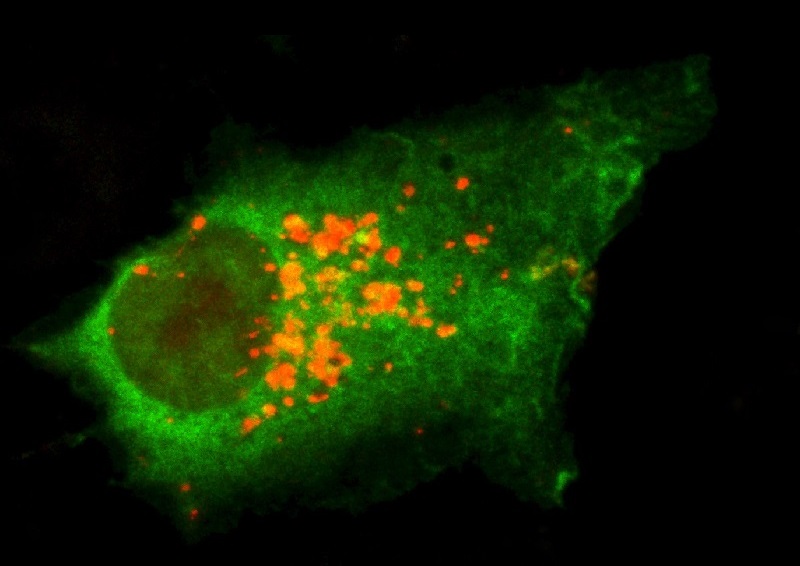
Autophagy Cleans up Macromolecular Damage
Our bodies have a nifty housekeeping service called autophagy that cleans up macromolecular damage. Unfortunately, the efficiency of our autophagy cell cleaning service declines with age.
Researchers have found that the drug rapamycin stimulates autophagy in lab animals, prodding our cells to clean out the toxic waste. You can learn more about this in next part of this series.
This was part one of a two-part series. Part 2 on autophagy and rapamycin is coming soon. Stay tuned.
Show Us Some Love
- One click helps us spread the word – Show some love and share this post on your social media account and share it with your friends. It only takes one click on any of the social media links on this page.
- Follow us on social media – For more articles, follow us on Google+ | Facebook | Reddit
- Sign up for our email list – We use your email to notify you of new articles. We won’t spam you, and we won’t share your email address. Cancel at any time.
- Tell us what you think – We love comments. Scroll down and leave your comments below.
References and Additional Reading
General References
Carlos López-Otín, et al. The Hallmarks of Aging. (2013) Cell, Volume 153, Issue 6, 1194 – 1217. Available Online.
Arlan G. Richardson, Eric E. Schadt; The Role of Macromolecular Damage in Aging and Age-related Disease, The Journals of Gerontology: Series A, Vol. 69, Issue Suppl_1, 1 June 2014, Page(s) S28–S32, https://doi.org/10.1093/gerona/glu056
AGEs References
Su-Yen Goh, Mark E. Cooper; The Role of Advanced Glycation End Products in Progression and Complications of Diabetes, The Journal of Clinical Endocrinology & Metabolism, Volume 93, Issue 4, 1 April 2008, Pages 1143–1152, https://doi.org/10.1210/jc.2007-1817
Woltjer, Randall L., Maezawa, Izumia, O, Joyce J, Montine, Kathleen S., Montine, Thomas J. “Advanced glycation endproduct precursor alters intracellular amyloid-beta/A beta PP carboxyterminal fragment aggregation and cytotoxicity.” Journal of Alzheimer’s Disease, vol. 5, no. 6, pp. 467-476, 2003. DOI: 10.3233/JAD-2003-5607. Available Online.
Ravichandran Ramasamy, Susan J. Vannucci, Shirley Shi Du Yan, Kevan Herold, Shi Fang Yan, Ann Marie Schmidt “Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation” Glycobiology, Volume 15, Issue 7, 1 July 2005, Pages 16R–28R, https://doi.org/10.1093/glycob/cwi053
Lipotoxicity References
Marc Slawik, Antonio J. Vidal-Puig, “Lipotoxicity, overnutrition and energy metabolism in aging,” In Ageing Research Reviews, Volume 5, Issue 2, 2006, Pages 144-164, ISSN 1568-1637, https://doi.org/10.1016/j.arr.2006.03.004. Article Link.
Wen Guo, Tamar Pirtskhalava, Tamara Tchkonia, Weisheng Xie, Thomas Thomou, Jianrong Han, Tong Wang, Siu Wong, Andrew Cartwright, Fausto G. Hegardt, Barbara E. Corkey, and James L. Kirkland. “Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity.” American Journal of Physiology – Endocrinology and Metabolism. Published 1 April 2007 Vol. 292 no. 4, E1041-E1051 DOI: 10.1152/ajpendo.00557.2006. Article Link.
Disclaimer
Diagnosis, Treatment, and Advice: This article is intended for educational and informational purposes only and is not a substitute for qualified, professional medical advice. The information and opinions provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. Experimental therapies carry a much higher risk than FDA-approved ones. Consult a licensed and qualified physician for the diagnosis and treatment of any and all medical conditions. Call 911, or an equivalent emergency hotline number, for all medical emergencies. As well, consult a licensed physician before changing your diet, supplement or exercise programs. Photos, Endorsements, & External Links: This article is not intended to endorse organizations, companies, or their products. Links to external websites, mention or depiction of company names or brands, are intended for illustration only and do not constitute endorsements.
14 Replies to “Macromolecular Damage Ages Us Prematurely – Autophagy Helps”
Comments are closed.