Can We Reverse Stem Cell Decline and Rejuvenate Our Bodies? (Part 2)
Summary: Our stem cells decline with age leading to decreased ability to repair our tissues. Fortunately, a pool of ‘lazy’ stem cells remains in our aging bodies. Given the right growth factors, these lazy stem cell can be woken up to repair our aging organs and tissues. This is part 2 of a two-part series – part 1 of the series on stem cell decline is here. This article first appeared on LongevityFacts.com, follow us on Google+ | Facebook | Reddit. Author: Brady Hartman.
Most of us have noticed that injuries in older people heal more slowly than in younger ones. For example, a fractured bone in an elderly person takes much longer to recover than in a younger person. This decline in healing ability is a characteristic hallmark of aging and applies to most of the tissues in our bodies. The reason for our deteriorating repair capabilities is due to a decline in the abilities of our stem cells, the repairmen of our bodies. We still have stem cells in old age, but they are not working the way the did in our youth.
There is good news, however. Geroscientists have recently discovered several possible causes of stem cell decline. Even better yet, they think they have found a way to wake up the dormant stem cells in our aging bodies.
Stem Cell Decline
Leading geroscientists implicate stem cell decline as a significant source of dysfunction and aging. In his landmark review, The hallmarks of aging, author Carlos López-Otín names ‘stem cell exhaustion’ as one of the nine most important characteristics of aging. As López-Otín states in his oft-quoted review,
“Stem cell exhaustion unfolds as the integrative consequence of multiple types of aging-associated damages and likely constitutes one of the ultimate culprits of tissue and organismal aging.“
How to Reverse Stem Cell Decline?
One way to rejuvenate our bodies is to reverse stem cell decline. This raises two questions:
- What causes stem cell decline? Is it due to the decrease in stem cell numbers, a reduction in their functional abilities, a degradation in their microenvironment or all of the above?
- How can we reverse stem cell decline?
Stem Cells as Repairmen
To understand the impact of stem cell decline, one must first understand the role that stem cells play in the repair process and also understand the lifestyle of a stem cell. Stem cells play different roles and change their abilities as they age.
Adult Stem Cells
Adult stem cells can remain dormant for long periods, change at will into various cell types to replace tissues and have a tendency slow down with age. Adult stem cells are multipotent – meaning they can in different kinds of specialized cell lines. For example, mesenchymal stem cells can become many different types of cell lines, including fat, bone, muscle, cartilage or neuronal cell lines.
Self-Renewal and Commitment
Adult stem cells are capable of self-renewal, but unlike true stem cells, are not capable of forming an entire embryo and placenta. Not capable that is, without genetic modification. Adult stem cells reside in protected niches in nearly all tissues. They infrequently divide unless the body needs to generate new progenitor cells during rapid tissue turnover or after injury.
Life is Different for a Progenitor Cell
Progenitor cells are stem cells that can renew themselves and are committed to developing into a specialized type of cells, such as brain or bone. Said in another way, a progenitor cell is a particular type of stem cell that can transform into a specific tissue but is more confident as to its future career path than other types of stem cells. This is why sometimes progenitor cells are referred to as being committed.
While the terms ‘progenitor cell’ and ‘stem cell’ are sometimes equated, there are differences between the two. A critical difference between progenitors and run of the mill stem cells is that the latter can replicate indefinitely, while progenitors can divide only a limited number of times.
Multipotent Stem Cells Are Potent Indeed
Multipotent adult stem cells can become committed to particular lineages through determination, a process which is mediated by external and internal signals and hormonal, paracrine, and metabolic factors. Even if adult stem cells can proliferate and produce committed progenitor cells able to differentiate, this process may produce a skewed population of progenitors. For example, some adult cells may produce a progenitor cell population that is skewed toward the myeloid lineage with aging in the hematopoietic system, the bodywide function that controls our blood and immune system.
Stem Cells In The Repair Process
The body responds to injury by dialing up cell turnover and increasing the replication of committed progenitor cells. The damage response team also ramps up the differentiation of progenitor cells into specialized cells, and increases the recruitment of new stem cells, committing the recruits to the repair mission at hand. The repair process also accelerates cell arrest and cell death, including the processes of senescence, necrosis, and apoptosis as it regenerates damaged tissues.
Cell Turnover Rates
The cells in our organs turn over throughout our lifespan, including those in the brain and heart. Cell turnover rates vary considerably depending on the tissue, with some cells being replaced at a high rate, like the staff at a fast-food restaurant, and others not turning over very much at all, as if they had a government job.
Turnover rates vary considerably among the different tissues, with gut epithelial cells being replaced every few days, skin every few weeks, red blood cells every few months, and adipose tissue in our ever-expanding mid-sections being replaced every few years. Competing for the last place are the cardiomyocytes, the cells that power our heart muscles. These hard-working cells turn over only once or twice in a lifetime.
That is why it pays to take care of your heart.
Stem Cell Decline in Aging
There is no doubt that stem cells decline during aging. This decline is responsible for the observation that it takes older individuals longer to heal than younger ones.
Declining Tissue Repair
The basis for this decline is due to dynamic changes in stem and progenitor cell function, including reductions in these cell’s capacities for replication and full differentiation into specialized types, as well as changes in propensities for senescence, apoptosis, and necrosis. The changes to a person’s stem cell throughout his lifespan are detailed in the following sections.
Differences in Differentiation
As we age, our progenitor cells are increasingly less able to differentiate into other types. These differences in differentiation are most apparent when comparing young progenitors with old ones.
On average, colonies of cells derived from single progenitors isolated from older animals undergo fewer divisions than clones isolated from younger ones. Despite this general rule, some individual clones from older animals can behave like those from younger ones, and vice versa.
Unfortunately, as we age, the capacity of our committed progenitor cells to differentiate into specialized cell types declines in several tissues, such as adipose tissue. For evidence, one only has to examine cell colonies derived from single progenitor cells taken from older animals and compare them to ones taken from younger individuals. Despite this, as concerns age-related decline in replicative potential, some clones that initially came from older individuals behave like cells taken from younger ones.
Researchers have linked impaired differentiation with decreased signaling through the transcription factor cascades that regulate the differentiation process. This may be due to age-related increases in inflammatory mediators, as it appears that this contributes to declines in the cell’s capacity for differentiation, at least in the case of adipose tissue.
Progenitor Decline Begets Disease
The decreased capacity of fat cell progenitors to differentiate into proper fat cells may contribute to age-related insulin resistance and diabetes. In turn, these conditions produce even more chronic inflammation, thus perpetuating a cycle. For evidence of this effect, one only has to look at undifferentiated progenitors whose numbers tend to increase relative to specialized cells as we age.
This effect is most notable in adipose tissue and tissues of the gut.
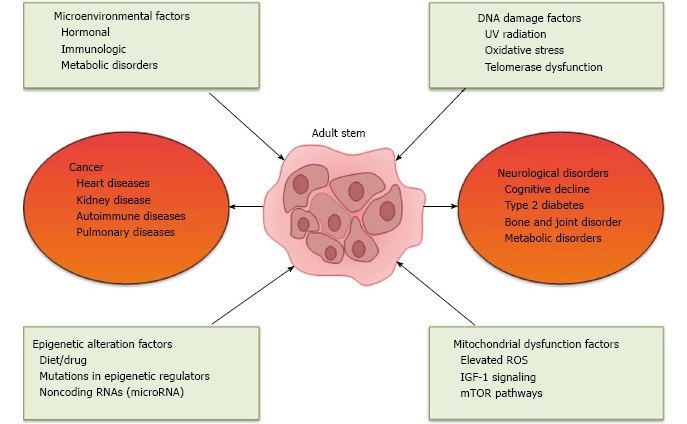
Reasons for Stem Cell Decline
Unfortunately, stem cell and progenitor pools are limited and can become depleted with age.
In a review published early this year in the World Journal of Experimental Medicine. Authors Abu Shufian Ishtiaq Ahmed and colleagues presented a list of suspects implicated in stem cell decline. The chief categories of stem cell assailants include microenvironmental changes, epigenetic changes, DNA damage and mitochondrial dysfunction. As well, Ahmed & co implicate chronic diseases as participating in the downfall of stem cells.
Stem Cells Are Cells Too
The list of things that age stem cells appear very similar to the list of things that age regular cells. For example, in his landmark review, leading geroscientist Carlos Lopez-Otin blames epigenetic changes, mitochondrial dysfunction and DNA damage as three of nine hallmarks of an aging characteristic of regular cells. It makes sense that these factors would also plague stem cells, for, after all, they are also cells.
Bad Neighborhood (Stem Cell Niche Decline)
Non-autonomous changes in the stem cell niche, also called the microenvironment, are partly to blame for the decline in the recruitment of adult stem cells. Chronic low-grade inflammation brought about by aging may also contribute. This chronic inflammation creates a toxic environment that impedes proper stem cell function. As well, cross-talk between organs can affect progenitor cell function, and this communication becomes dysfunctional with increasing age. Examples of this include the cross-talk between the adipose tissue and those in the bone.
Stem Cell Decline Due a Limited Supply of Silver Bullets
Although not mentioned in the review by Ahmed & Co, another reason for stem cell decline may be that we simply use them too much. In other words, one reason for the decline may be due to increased utilization of stem cells for tissue repair. During aging, autonomous and non-autonomous cell changes can occur that have the effect of restricting the cells ability to replicate which in turn, interferes with the repair process following injury or disease.
Possible Ways To Rejuvenate Stem Cells
Ahmed & Co proposed several ways to rejuvenate stem cells, including Parabiosis (changing the microenvironment), telomere lengthening, reprogramming regular cells into iPSCs, and retrotransposons.
Of the items on their list, changing the microenvironment seems to be the low-hanging fruit, and lengthening telomeres seems promising.
Telomere Lengthening
Unlike adult stem cells, committed progenitors usually do not express the telomere-repairing enzyme called telomerase. Because of this and other factors, committed progenitor cells have limited replicative potential in the laboratory. Over the course of its lifetime, the cumulative replicative history of a progenitor increases, and its telomere length decreases.
Partly related to this shortened lifespan, progenitor cells isolated from the marrow, skin, fat, and other tissues of older animals have an overall reduced capacity for further proliferation when compared to cells from younger ones.
Ahmed & Co suggest lengthening telomeres as a way to revitalize stem cells. While tinkering with the telomeres of healthy cells seems a bit risky, geroscientists are making headway. In fact, this year, Dr. John Cooke lengthened telomeres using RNA therapy and succeeded in reversing aging in human cells.
Changing The Microenvironment To Rejuvenate Stem Cells
As recent experiments show, improving the niche or microenvironment of a stem cell can revitalize a stem cell. This year, a researcher revitalized aging blood stem cells by merely adding the proper growth factor.
Revitalizing the stem cell niche also seems the most straightforward intervention. All researchers have to do is find the right growth factor and turn it into therapy.
Parabiosis Experiments Show Importance of the Niche
For proof that changes in the microenvironment affect stem cell function, there is no better example than parabiosis experiments. Often referred to as the ‘young blood old blood’ phenomenon, parabiosis experiments consist of surgically joining the circulatory systems of old and young mice so that their blood commingles for several weeks or months.
Be assured that the mice are not too happy about this experiment.
Circulating Factors – Share and Share Alike
When the older mouse sustains heart or skeletal muscle injury, circulating factors from the young rodent hasten repair. This repair is performed by the older mouse’s progenitor cells. Likewise, under the principle of share and share alike, blood factors from the old rodents impede progenitor function and tissue repair in the younger mice.
The results of parabiosis experiments that circulating factors – whether from inflammation, cellular senescence, or paracrine factors – appear to be important drivers of age-related dysfunction of adult stem cells. In other words, aging progenitor cells may be in fine working order, and their only problem is that their function has been suppressed by an aging microenvironment.
Cleaning Up the Neighborhood
By supplying old mice with circulating growth factors obtained from younger ones, researchers believe that by restoring progenitor cell function, they can restore the function of an old mouse’s heart, muscle, and brain.
Geroscientists speculate that the key rejuvenating factor is Growth and Differentiation Factor 11 (GDF-11). However, the role of GDF-11 as the fountain of youth is subject to considerable controversy.
While this is good news for mice, what about for humans? The parabiosis experiments suggest that scientists can develop drugs based on these growth factors and these therapies could rejuvenate the bodies of aging humans.
Bottom Line
In conclusion, the ability of our progenitor cells to replicate and differentiate decreases with age. This decline may contribute to the body’s decreased ability to repair damaged tissue. Notwithstanding the preceding, a pool of stem cells remains in the aging body, and it contains functional progenitor cells that can repair tissues, particularly if researchers can tweak the microenvironment to make it more favorable.
Researchers have developed potential ways to improve the microenvironment in experimental animals. If they can perfect these techniques and turn them into interventions for humans, then they will have created a fountain of youth of sorts.
Related Articles
- Video – Can These Revolutionary Technologies Beat Aging in Our Lifetimes?
- Stem Cell Primer – What You Need to Know
- Can We Reverse Stem Cell Decline and Rejuvenate Our Bodies? (Part 1)
- Breakthroughs Set To Revolutionize Stem Cell Therapy.
- Scientists use human stem cells to grow functioning kidney tissue that produces urine.
-
Researchers report a breakthrough using stem cells in human lung regeneration.
Help Us Spread the Word
Please share this post and help us spread the word. All it takes is one simple click on any of the social media links on this page.
References
Cover Photo: Stem Cells. Credit: Allen Institute for Cell Science. Allen Cell Explorer Launched (press release dated Apr 05, 2017). (adapted). Available Online.
López-Otín, Carlos et al. “The Hallmarks of Aging.” Cell 153.6 (2013): 1194–1217. PMC. Web. 8 Oct. 2017. Available Online.
Ahmed, Abu Shufian Ishtiaq et al. Effect of Aging on Stem Cells. World Journal of Experimental Medicine 7.1 (2017): 1–10. PMC. Web. 7 Oct. 2017. Available Online.
Tchkonia T, Morbeck D.E., von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging Cell 9:667–684, 2010.
Michael B. Schultz & David A. Sinclair. “When stem cells grow old: phenotypes and mechanisms of stem cell aging.” Development. 2016 Jan 1; 143(1): 3–14. doi: 10.1242/dev.130633 PMCID: PMC4725211. Available Online.
Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nature Cell Biology 13:506–512, 2011.
Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764, 2005.
Gimble JM, Nuttall M.E. The relationship between adipose tissue and bone metabolism. Clinical Biochemistry 45:874–879, 2012.
Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344:649–652, 2014.
Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proceedings of the National Academy of Science. 102:9194–9199, 2005.
Kirkland JL, Hollenberg CH, Gillon W.S. Age, anatomic site, and the replication and differentiation of adipocyte precursors. American Journal of Physiology 258:C206–C210, 1990.
Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344:630–634, 2014.
Disclaimer
Diagnosis, Treatment, and Advice: This article is intended for educational and informational purposes only and is not a substitute for professional medical advice. The information and opinions provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. Consult a licensed and qualified physician for the diagnosis and treatment of any and all medical conditions. Call 911, or an equivalent emergency hotline number, for all medical emergencies. As well, consult a licensed physician before changing your diet, supplement or exercise programs. Endorsements, Photos, and External Links: This article is not intended to endorse organization, companies, or their products. Links to external websites, mention or depiction of company names or brands, are intended for illustration only and do not constitute endorsements.
12 Replies to “Can We Reverse Stem Cell Decline and Rejuvenate Our Bodies? (Part 2)”
Comments are closed.