Can These Novel Treatments Cure Alzheimer’s Disease?
Over 100 potential Alzheimer’s disease treatments are in the pipeline, using novel approaches that may reduce the number of people affected by about 40 percent, report industry experts. [This article first appeared on LongevityFacts and was updated on March 21, 2018. Author: Brady Hartman. ]
Scientists are in a race to be the first to develop a cure for Alzheimer’s disease and are approaching the problem in revolutionary ways.
It’s not just novel compounds that give scientists high hopes for a cure, but the revolutionary ways in which researchers and industry are tackling the problem.
Expecting the sizable payoff that awaits the first one to cure Alzheimer’s, pharmaceutical companies are taking much higher risks and investing far more than with other conditions. Moreover, despite early failures with specific compounds, they continue to test these compounds at different doses or with patients who have different characteristics. This is a revolutionary change for an industry that is quick to abandon a potential drug at the first sign of trouble.
What’s more, Alzheimer’s treatment developers are targeting people at earlier and earlier stages – testing therapies on patients even before they have symptoms of the disease.
There are nearly 100 potential medicines for Alzheimer’s in trials. Credit: PhRMA.
Alzheimer’s Treatments in the Pipeline
Advancements in science combined with these novel approaches have yielded a pipeline full of potential Alzheimer’s treatments along with the funds to test them. According to this year’s report by PhRMA, the Pharmaceutical Research and Manufacturers of America (PhRMA), there are
“100 potential medicines in clinical trials “
The hundred potential treatments in the pipeline range from the use of MRI technology for early detection of Alzheimer’s to antibodies that attack beta-amyloid, to therapies that target tau protein tangles. With all these bets, scientists are optimistic that a cure will be found well within our lifetime.
Expect the number of compounds in clinical trials to grow as researchers discover new targets every day. Just a few days ago, researchers at King’s College London found a key mechanism in neurodegenerative diseases like Alzheimer’s.

What is Alzheimer’s?
Alzheimer’s disease is a form of dementia – a collective term for brain disorders, which affect emotion, memory, thinking, and behavior. Due to physical changes in the brain, dementia leads to the gradual deterioration in our ability to think and remember things.
All of us experience aging in our brain. Starting from the age of 40, the average brain will shrink at a rate of 5% per decade, and accelerate after the age of 70.
As we get older, our odds of getting Alzheimer’s or any other form of dementia increase with every birthday. In fact, some form of dementia affects about half of the population age 85 years or older.
Dementia is the leading cause of disability among the aged, and it’s a huge problem, and an April 2017 report by the World Health Organization (WHO), says
“A new case of dementia is diagnosed every 4 seconds.”
While there are over 100 forms of dementia, Alzheimer’s disease is the most common form and accounts for around 60% of all cases. Other forms of dementia include frontotemporal dementia, vascular dementia, and dementia with Lewy bodies.
Early detection and treatment could be the way to cure Alzheimer’s. Unfortunately, almost all cases of dementia are detected after the patient starts to display physical symptoms of the disease, by which time it has already progressed, and parts of the brain have been irreparably damaged.
Symptoms of dementia may include personality and mood changes, loss of memory, difficulty in performing previously routine tasks and difficulty in finding the right words or understanding what people are saying.
Alzheimer’s and other forms of dementia are massive problems that are about to get a whole lot bigger, according to the April report by WHO that says,
“The number of people living with dementia worldwide is currently estimated at 47 million and is projected to increase to 75 million by 2030. The number of cases of dementia are estimated to almost triple by 2050. ”
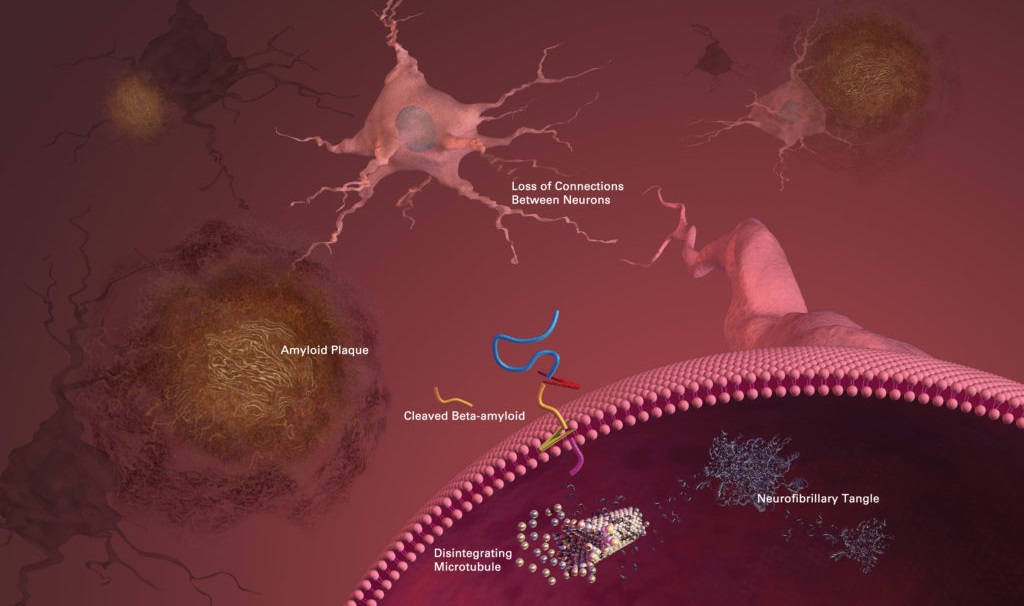
The Processes Underlying Alzheimer’s
Researchers are not entirely sure what causes Alzheimer’s disease, however, according to the Alzheimer’s Association, beta-amyloid plaques and tau protein tangles are prime suspects in the loss of function and cell death in the brains of patients.
In part, Alzheimer’s and other neurodegenerative diseases are caused by a build-up of debris in the brain – a phenomenon known in the scientific literature as cellular garbaging, a term coined by the highly regarded geroscientist Claudio Franceschi.
One example of this cellular garbage – also called intracellular junk by Aubrey de Grey of SENS – are the tau protein tangles found inside the brain cells of Alzheimer’s patients. Also called neurofibrillary tangles, these are twisted fibers of a protein called tau. In healthy neurons, tau forms part of a structure called a microtubule which helps transport nutrients from one part of the brain cell to another. However, in areas with tangles, the twisted strands of tau disable the supply system so that nutrients can no longer move throughout the cells.
A study by Chesser, Pritchard, and Johnson shows that this build-up of tau protein tangles is caused by a failure of the cellular housekeeping process known as autophagy which fails to clear damaged tau fragments. In fact, the loss of autophagy affects many other areas in our bodies and is one of the nine hallmarks of aging common to all us.
Another prime suspect in Alzheimer’s disease is the beta-amyloid plaques found in the brain cells of patients. These plaques are a type of extracellular junk and thought to play a substantial role in Alzheimer’s disease. These are clusters of sticky proteins called beta-amyloid that abnormally build-up outside of brain cells. The most damaging form of beta-amyloid may be groups of a few pieces rather than the large plaques. These small clusters may block signaling at the synapses and may also trigger chronic inflammation and activate immune system cells that devour disabled cells. In any disease, inflammation is usually a sign of tissue damage.
Scientists believe that the accumulation of aggregated amyloid fibrils induces a form of programmed cell death called apoptosis. Researchers have also found that beta-amyloid builds up in the mitochondria in the brain cells of Alzheimer’s patients, and inhibits some enzyme functions and glucose utilization by neurons.
Though most people develop some beta-amyloid plaques and tau protein tangles as they age, those with Alzheimer’s tend to develop far more. The plaques and tangles tend to form in a predictable pattern, starting in areas involved in learning and memory and then spreading to other regions.
Can Novel Treatments Cure Alzheimer’s?
The hundred Alzheimer’s treatments in the pipeline range from early detection to novel therapies that attack beta-amyloid and tau protein tangles. Many of the new treatments being developed have the potential to be the first disease-altering medication for Alzheimer’s.
The more scientists learn, the better they are at developing novel treatments aimed at slowing, and even preventing Alzheimer’s entirely. In the past decade, advancements in science have helped researchers unravel some of the significant complexities of Alzheimer’s disease and other forms of dementia.
Beta-Amyloid Therapies
The primary strategy to cure Alzheimer’s involves removing beta-amyloid aggregates. Most of the clinical trials are devoted to testing the hypothesis that reducing beta-amyloid accumulation in the brain will prevent or slow the progression of memory problems. So far, clinical trials of compounds that target beta-amyloid have only resulted in a series of high-profile failures.
Despite the clinical trial failures, the case for removing beta-amyloid is strong. A mutation in beta-amyloid in some Icelanders reduces its levels by half throughout their lives. These fortunate Icelanders virtually never develop Alzheimer’s disease.
Giving the high-profile failures, many observers are beginning to doubt the beta-amyloid angle. For example, authors of a recent opinion piece on Alzheimer’s treatments convincingly argue that clearing out amyloids starting at age 65 is too late to begin treatment. In fact, the authors suggest that clearing out beta-amyloid as a mono strategy may not be the answer.
The possibility that these authors may be right has led Alzheimer’s researchers to pursue other therapeutic strategies.
Clearing Out Tau
Scientists are also testing therapies that target the tau protein tangles that damage and kill brain cells. New strategies involving drugs or antibodies that reduce levels of defective tau protein are entering phase II trials.
Immunotherapies
Active and passive immunotherapies also hold promise. The term ‘active immunotherapy’ simply means vaccination. Researchers have yet to invent a vaccination that guards against Alzheimer’s.
Passive immunotherapies are comprised of antibodies or other immune system components that are made in the laboratory and then administered to patients to provide immunity. Passive immunotherapies using anti-tau antibodies are promising, and some are already in clinical trials. However, we won’t know the results of these trials until around 2022.
For example, one promising treatment involving passive immunotherapy uses an antibody aimed at directly attacking Alzheimer’s disease and prevent it from progressing. Another approach involves the use of antibodies to significantly reduce the level of the beta-amyloid protein found in the brains of individuals with early-onset Alzheimer’s.
Other Strategies
Moreover, researchers are testing treatments that target the receptor that decreases a neurotransmitter necessary for the brain to think and function normally.
Reducing Inflammation
Furthermore, pharmaceutical companies are designing drugs that reduce the chronic inflammation found in the brains of Alzheimer’s sufferers while strengthening the immune system to fight the disease.
Early Detection Approach
A novel approach uses imaging technology to detect Alzheimer’s before it has a chance to do damage. With this technique, researchers can now identify early physical symptoms, like plaque buildup, that may differentiate mild cognitive impairment related to early-onset Alzheimer’s from healthy aging. You’ll learn more about this approach later on, as it allows doctors to detect dysfunction in the brains of patients before they lose tissue and nerve cells.

Prospects for an Alzheimer’s Treatment
Given the truckload of potential treatments for Alzheimer’s, PhRMA reports that,
“If just one of these treatments is proven effective, we can possibly delay this disease by five years, reducing the number of people affected by roughly 40 percent.”
Can Early Detection Stop Alzheimer’s?
The early detection approach – such as that reported by PhRMA – makes a lot of sense. Scientists can monitor beta-amyloid deposits in the brain via an imaging technique called amyloid positron emission tomography (Amyloid PET), also known as ‘amyloid brain scanning’ and ‘amyloid imaging.’
The problem with current Alzheimer’s disease prevention trials is that they begin too late, usually with people around the age of 60 and only those people who are already showing worrisome accumulation of beta-amyloid as demonstrated by brain scans are admitted into the trial. For example, consider the current prevention trial called ‘A4’, which stands for “Anti-Amyloid Treatment of Asymptomatic Alzheimer’s.” A4 is a series of randomized trials of compounds that reduce the accumulation of beta-amyloid in the brain and only admits people who are 65 or older and have positive brain amyloid scans. Observers feel that the treatment is starting too late.
On the theory that early detection and treatment is a better way to cure Alzheimer’s, some pharmaceutical companies are starting the trial therapies in younger patients. In March of this year, Bloomberg magazine interviewed Phyllis Ferrell, who leads Eli Lilly’s Alzheimer’s program. Ferrell explained the reasoning behind the shift in focus to early detection of Alzheimer’s, saying
“It’s like cancer,” … “You want to catch it at stage one, not at stage four.”
Ferrell is optimistic that scientists will find a way to slow down Alzheimer’s, and also tells Bloomberg,
“We’re not going to have a reversible cure any time in the near future,” adding, still “I think in our lifetime we will have something that slows this disease.”
Gilmore O’Neil, an executive in clinical development at Biogen, also supports the early detection hypothesis. Bloomberg also interviewed O’Neil, who said:
“many industry studies so far have looked at patients who were already so sick that they’d probably be beyond saving.”
Many pharmaceutical companies are testing therapies on patients in early stages of the disease, sometimes including people who merely have brain scans showing beta-amyloid plaques and aren’t showing signs of forgetfulness at all.
And while the early detection approach makes sense, some experts disagree. In a recent opinion piece on finding a cure for Alzheimer’s, a group of authors affiliated with Mount Sinai said that for early treatment to be effective, it should be initiated at around age 55, and would subject patients to side effects for possibly no benefit.
Bottom Line
There is no known cure for Alzheimer’s. Existing treatments slightly slow symptoms at best. The possibility that clearing out amyloid plaques will not work, has led researchers to pursue other therapeutic strategies. The upshot of all of this is that we have more potential treatments than ever before and one of these might just defeat this deadly disease.
Other Articles in this Series
Part 2: Scientists propose precision treatments for Alzheimer’s disease.
Part 3: Researchers report on drugs that may prevent Alzheimer’s.
Additional Alzheimer’s Reports
- Researchers discover diabetes drugs as potential Alzheimer’s treatment.
- Scientists discover key mechanism in Alzheimer’s disease.
Show Us Some Love
Share this post on social media and help us spread the word– It only takes one click on any of the social media links on this page.
Follow us on social media – Google+ | Reddit
Sign up for our email list – We use your email to notify you of new articles. We will not share your email address or send you spam. You can cancel at any time.
Tell us what you think – Scroll down to enter your comments below.
References
Cover photo: Getty Images.
Van Broeck B, Van Broeckhoven C, Kumar-Singh S. “Current Insights into Molecular Mechanisms of Alzheimer Disease and Their Implications for Therapeutic Approaches.” Neuro-Degenerative Diseases. 2007;4(5):349–365. doi:10.1159/000105156. PMID 17622778. Link.
Huang, Yadong, and Lennart Mucke. “Alzheimer Mechanisms and Therapeutic Strategies.” Cell 148.6 (2012): 1204–1222. PMC. Web. 25 Nov. 2017. Link.
Xi Chen, Shi Du Yan. “Mitochondrial Abeta: A Potential Cause of Metabolic Dysfunction in Alzheimer’s Disease.” IUBMB Life. 2006;58(12):686–94. doi:10.1080/15216540601047767. PMID 17424907. Link.
Chesser, Adrianne S., Susanne M. Pritchard, and Gail V. W. Johnson. “Tau Clearance Mechanisms and Their Possible Role in the Pathogenesis of Alzheimer Disease.” Frontiers in Neurology 4 (2013): 122. PMC. Web. 24 Nov. 2017. Link.
Sam Gandy, Tamas Bartfai, Graham Lees, Mary Sano. “What Would It Take to Get an Effective Alzheimer’s Drug?” Scientific American. July 17, 2017. Web. Retrieved 25 Nov. 2017. Link.
Heneka MT. “Neuroinflammation in Alzheimer’s disease.” Lancet Neurology. April 2015. 14 (4): 388–405. doi:10.1016/S1474-4422(15)70016-5. PMID 25792098. Link.
Disclaimer
Diagnosis, Treatment, and Advice: This article is intended for educational and informational purposes only and is not a substitute for qualified, professional medical advice. The information and opinions provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. Experimental Alzheimer’s treatments carry a much higher risk than FDA-approved ones. Consult a licensed and qualified physician for the diagnosis and treatment of any and all medical conditions. Dial 9-1-1, or an equivalent emergency hotline number, for all medical emergencies. As well, consult a licensed, qualified physician before changing your diet, supplement or exercise programs. Photos, Endorsements, & External Links: This article is not intended to endorse organizations, companies, or their products. Links to external websites, mention or depiction of company names or brands, are intended for illustration only and do not constitute endorsements.

As regards Alzheimer’s disease, there are only TWO things you need to know. One is your family history of AD and the other is your APOE genetic status. A few people have APOE2, best, most have APOE3, very good and about 20% have APOE4. If you are carrier for APOE4, you are at high risk for AD, about 3-4 times the risk. Worse, if family history of AD in first degree relative and APOE4 carrier, may have 6 fold the risk. If family history and not APOE4 carrier, no increased risk. And the really bad news for APOE4 carriers, your median age of onset in some studies is about 75 compared to 85 if not APOE4 carrier.
So if not APOE4 carrier, cross AD off your list of things to worry about.
If carrier of APOE4, for starters, exercise and don’t overeat. Excess calories only big risk factor for APOE4 carriers.
Question: If APOE4 such a huge deal, why don’t the AD groups talk about APOE4.
Remember Jack Nicholson in “A Few Good Men” as the Colonel who very loudly testifies in courtroom, “You can’t handle the truth”. That’s the answer.
yes, it’s perplexing that NIA has spent hundreds of millions to identify these genetic risk factors, yet do not recommend a preventative course of action. Your tax dollars at work.