When Cell Death Goes Bad – Researchers Discover Role in Cancer and Inflammation-linked Diseases
Summary: Programmed cell death is an essential part of staying healthy. However, when the process goes haywire, it promotes the growth of cancer and inflammation-related diseases such as such as rheumatoid arthritis, atherosclerosis, systemic lupus erythematosus (SLE), sepsis, and diabetes. A recent review highlights the role played by an out-of-whack cell death system in cancer and inflammation-related diseases. [Cover: Dr. Microbe / Getty Images (adapted).]
Cell death is essential to our health. However, when the process goes haywire, it puts our health at stake.
Two recently published reviews shed new light on cell death and the role it plays in cancer and inflammation-related diseases, such as atherosclerosis, systemic lupus erythematosus (SLE), rheumatoid arthritis, sepsis, and diabetes.
The research performed by a collaboration between scientists at the University of Washington in Seattle (UWS) and the National Institute of Environmental Health Sciences (NIEHS), shows that when the cell death process gets out of whack, it enables cancer to spread and leads to neurodegenerative diseases. Publishing their findings in October of this year in the journal Trends in Immunology, the authors of the research article implicate ‘cell death gone haywire’ in many inflammation-related diseases, such as rheumatoid arthritis, systemic lupus erythematosus (SLE), sepsis, atherosclerosis, and diabetes. The authors of the UWS-NIEHS study, Joseph P. Kolb, Thomas H. Oguin III, Andrew Oberst, Jennifer Martinez highlight the importance of their findings, saying
“Whereas the most common form of programmed cell death, apoptosis, is designed to be immunologically silent, other forms of programmed cell death, like necroptosis and pyroptosis, are lytic in nature and result in the release of damage-associated molecular patterns (DAMPs).”
However, the process can go wrong and lead to chronic inflammation and age-related disorders, as the authors add:
We now recognize that defects in efferocytosis [the way the immune system cleans up dead cells], including defects in the proper processing of ingested cellular corpses, can lead to inflammatory and autoimmune disorders.
Cell Death a Part of Life
Our body’s cells are in a perpetual cycle as they continually die and are replaced by new ones. This tightly regulated process called cell death is essential for normal function and removing damaged tissue. Our bodies need to keep a tight lid on the number of cells as well as get rid of the broken ones. Cell death is nature’s housekeeper and stops excessive and damaged cells from accumulating. As the UWS-NIEHS authors say,
“The life of an organism requires the assistance of an unlikely process: programmed cell death. “
Cell death is required for our normal health and is a choice that our cells have to make, sometimes voluntarily, other times accidentally. The disruption of the cell death mechanism can often contribute to cancer and other diseases.
The body aims to maintain balance, also called homeostasis. Without this balance, our health fails and disease increases. Cell death is also the body’s way of defending itself against pathogens, such as bacteria, viruses, and even toxins. Whenever a cell is damaged or infected with bacteria, cell death is the process used to cull it from the flock. The immune system then comes along to sweep up the mess.
The factors that can trigger cell death are diverse. The molecular mechanisms of cell death and cell survival are complex and often intertwined with other cellular machinery, such as that for cell proliferation, cell differentiation, thus casting a broadly connected signaling network.
Unfortunately, programmed cell death does not always work as planned, and when the system goes haywire, it leads to big trouble in the body. When the normal cell death and clearance process gets out of whack, it leads to diseases such as cancer, neurodegeneration, and autoimmune conditions.
Cell Death – Many Ways To Die
A cell can degrade in three principal ways, including autophagy, apoptosis, and necrosis. When a cell dies, it leaves a corpse which cannot stick around forever. The immune system acts as the body’s housekeeper and employs phagocytes, specialized white blood cells that engulf our dying cells.
Phagocytes continually patrol our tissues on the lookout for dying cells, who broadcast a “come get me” message. And if the dying cell is transmitting an “eat me” signal, then the phagocyte engulfs it. Cell death can be either pro- or anti-inflammatory, and phagocytes are the gatekeepers of inflammation.
Autophagy
Autophagy is considered a pro-survival mechanism, and is part of the cell’s stress response mechanism, helping the cell survive during lean times. Excessive autophagy, however, leads to cell death. Autophagy is part of the cell’s stress response and occurs when the cell is experiencing stress or is going through lean times. Autophagy literally means ‘eating oneself.’ Autophagy helps the cell to survive under stressful conditions such as a deficiency of nutrients. When a cell is starving, it can survive by digesting part of its insides.
Additionally, autophagy is used to counter cell stress, which occurs when the cellular machinery becomes damaged, or proteins stick together in messy clumps called misfolded proteins. In this case, autophagy removes the danger by digesting the culprits. Sometimes autophagy can become impaired, and this condition brings dire consequences.
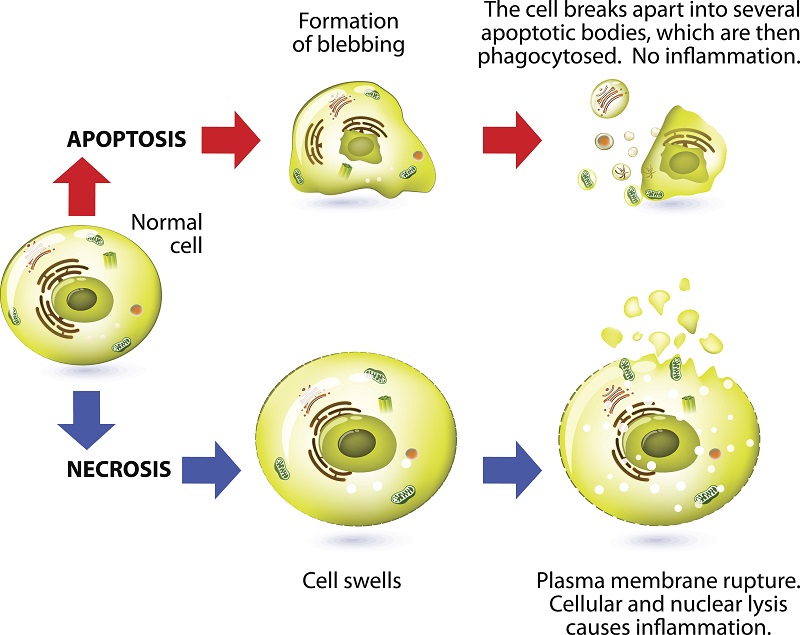
Cell Death via Apoptosis
Apoptosis or programmed cell death occurs as a healthy and controlled part of an organism’s growth and development. During apoptosis, a cell is broken up into small, self-contained pieces, which are easily recycled by phagocytes.
Apoptosis is often kick-started by an accumulation of stress signals, such as too many misfolded proteins, low oxygen or damaged DNA. As well, outside triggers can set off apoptosis by activating death receptors in the cell. To make it easy for patrolling phagocytes to find and kill apoptotic cells, the dying cells release both “come get me” and “eat-me” signals.
Once the apoptosis program starts, it cannot be turned off. The cell generates enzymes that destroy it from the inside. Then, a chain of biochemical events leads to other well-defined changes in cell characteristics, which eventually results in death for the cell.
Learn more about cell stress and apoptosis in this report.
Cell Death by Necrosis
When a cell dies by necrosis, it bursts and releases its guts into the surrounding tissue. Death by necrosis comes in two forms: passive and programmed.
Cell Death by Passive Necrosis
Conventionally, necrosis means a passive, unplanned death of the cell caused by cellular damage or pathogens, in contrast to orderly, programmed death via apoptosis. Necrosis happens in response to high pressure or high temperature. Researchers deem this the passive form of necrosis, as it does not require any specific activity by the cell.
Programmed Cell Death by Necroses
Necrosis can be intentional and actively regulated by the cell and is termed programmed cell death. As with passive necrosis, the cell swells until it bursts. However, it is not due to overt external sources, but rather a strictly orchestrated sequence of events. This category of programmed necrosis is in turn subdivided into two more categories: necroptosis, and pyroptosis.
Cell Death by Necroptosis
The first type of programmed necrosis is called necroptosis, or inflammatory cell death. As with passive necrosis, the cell swells until it bursts due to a carefully orchestrated sequence of events. When the cell bursts, it spills its contents into the surrounding space. Both necroptosis and pyroptosis promote inflammation to alert the immune system of pathogen infection. These components called damage-associated molecular patterns (DAMPs) put the immune system on alert.
Cell Death by Pyroptosis
The second type of programmed necrosis is pyroptosis, a highly inflammatory form of programmed cell death. Pyroptosis typically forms part of the antimicrobial response and occurs when the cell is infected with pathogens. In this process, immune cells recognize that they have invaders lurking within, release pro-inflammatory cytokines, swell, burst and die. They release cytokines which attract other immune cells to fight the infection, which in turn, stimulates inflammation in the surrounding tissue. Pyroptosis promotes the rapid clearance of various bacterial and viral infections by enhancing the host’s defensive responses.
Unfortunately, pyroptosis turns against us in chronic pathogenic diseases. In these conditions, the inflammatory response does not completely remove the primary stimulus, as typically occurs in most infections or injuries. As a result, chronic inflammation ensues that damages our tissues.
Chronic Inflammation Caused by Cell Death
The authors of the UWS-NIEHS study noticed a strong link between necroptosis and pyroptosis and inflammation-related diseases, such as atherosclerosis, systemic lupus erythematosus (SLE), rheumatoid arthritis, sepsis, and diabetes. Both necroptosis and pyroptosis generate bucketloads of DAMPs which inflame the surrounding tissue. DAMPs in the tissue act like blood in the water for sharks. In this case, the ‘sharks’ are the phagocytes and other immune cells. DAMPs cause them to spring into action and produce inflammation. DAMP-induced inflammation can lead to systemic, inflammation, which in turn, can provoke the chronic diseases of aging.
Researchers are starting to understand the link between necroptosis and inflammatory diseases, such as atherosclerosis. Researchers have implicated pyroptosis in the development of systemic lupus. As well, scientists have implicated necroptosis in Huntington’s disease, and Amyotrophic Lateral Sclerosis (ALS).
Cell Death Goes Awry in Cancer
Sometimes, there is not enough cellular suicide, especially in the case of cancer.
Tumors are masters at evading our immune system and avoiding cell death. When cancer cells spread to distant sites or metastasize, to stay alive, they must avoid the cell death pathways.
Cancers are talented at avoiding death and have sophisticated mechanisms that hijack components of the autophagy, apoptosis, and necrosis signaling pathways. When they spread through the body, cancers escape cell death by deactivating these pathways.
Bottom Line
One Click Helps Us Spread the Word
Please re-share this post and help us spread the word. It only takes one simple click on any of the social media links on this page.
References
Joseph P. Kolb, et al. Programmed Cell Death and Inflammation: Winter Is Coming. Trends in Immunology, Volume 0, Issue 0. DOI: http://dx.doi.org/10.1016/j.it.2017.06.009. Published online: July 19, 2017.
Nader Yatim, Sean Cullen, Matthew L. Albert. Dying cells actively regulate adaptive immune responses.
Nature Reviews Immunology. Nature Publishing Group. Mar 13, 2017. Link to article.
Rebecca SY Wong. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011; 30(1): 87. Published online 2011 Sep 26. doi: 10.1186/1756-9966-30-87. PMCID: PMC3197541. Link to article.
Disclaimer
Diagnosis, Advice, and Treatment: This article is intended for educational and informational purposes only and is not a substitute for qualified, professional medical advice. The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. Instead, consult a licensed physician for the diagnosis and treatment of any and all medical conditions. Call an emergency hotline such as 911, or an equivalent number, for all medical emergencies. Additionally, consult a licensed physician before changing your diet, supplement or exercise programs. Endorsements, Photos & External Links: This article is not intended to endorse specific organizations, companies, or their products. Links to external websites, mention or depiction of company names or brands, are intended for illustration only and do not constitute endorsements.
22 Replies to “When Cell Death Goes Bad – Researchers Discover Role in Cancer and Inflammation-linked Diseases”
Comments are closed.