Scientists Reveal Keys to Our Telomere Length (part 1 of 3)
Summary: An extensive new study on the role of telomere length in the chronic diseases of aging reports on the use telomere length as biomarkers of aging, in maximum human lifespan, and the wrenching choice between cancer and degenerative diseases such as respiratory diseases and dementia. This is part 1 of a three-part series. Part 2 on telomere length and cancer is here, Part 3 on telomere length and chronic diseases is here. [This article first appeared on the website LongevityFacts.com. Author: Brady Hartman. ]
This first segment reports what researchers discovered about the accuracy of telomere length studies, the role of telomeres in maximum human lifespan and the accuracy of using them as a biomarker of aging.
In a comprehensive study published a little more than a week ago in a highly respected journal of the Royal Society, authors Aviv and Shay nail down the answers to puzzling questions regarding telomere length and human aging and health. The Royal Society is the highly-respected national science academy of the United Kingdom, with a fellowship of the world’s most eminent scientists.
Authors Aviv and Shay perform an exhaustive review of the literature to provide a comprehensive look at the role of telomere length in chronic human diseases, such as cancer and the reliability of telomere length studies and their use as biomarkers.
Their article provides insight into the role of telomere length in human health and disease in the general population. In their study titled “Reflections on telomere dynamics and ageing-related diseases in humans,“ Aviv and Shay outline their agenda, saying
“Here we distil the essence of a large body of research to paint a broad picture of the role of telomeres in ageing-related human diseases.”
Aviv is with the Center of Human Development and Aging, at New Jersey Medical School at Rutgers, The State University of New Jersey. Shay is with the Department of Cell Biology at the UT Southwestern Medical Center in Dallas, and also with the Center of Excellence in Genomic Medicine Research at King Abdulaziz University in Saudi Arabia.
Overview of Telomere Series
Part 1 – Telomere Length (this article)
- Accuracy of Telomere Length Studies
- Using Telomere Length as a Biomarker of Aging
- Telomere Length Determines Maximum Human Lifespan
Part 2 – Cancer and Telomere Length
- Solving Richard Peto’s Paradox
- How Bowhead Whales avoid cancer
- Cancer and Telomeres
Part 3 – Telomere Length
- Telomeres As Biological Clocks
- Telomere Length and Chronic Diseases
Why Do We Need Telomeres Anyway?
Looking very much like the plastic tips on the ends of shoelaces, telomeres are the protective caps on the ends of all human chromosomes that protect our genetic data. Broken chromosomes cause all sorts of trouble and trigger ill health.
Moreover, telomeres act as a biological clock in our cells. In healthy aging, each time a cell divides, its telomeres get shorter, until the cell either becomes senescent or undergoes a form of cell death.
Geroscientists link inherited short telomeres to a variety of human disorders, including pulmonary fibrosis, aplastic anemia, dyskeratosis congenita, and cancer. In addition to these rare genetic disorders, researchers link short telomeres in the general population to several common chronic diseases, such as diabetes, cardiovascular diseases, hypertension, and dementia.
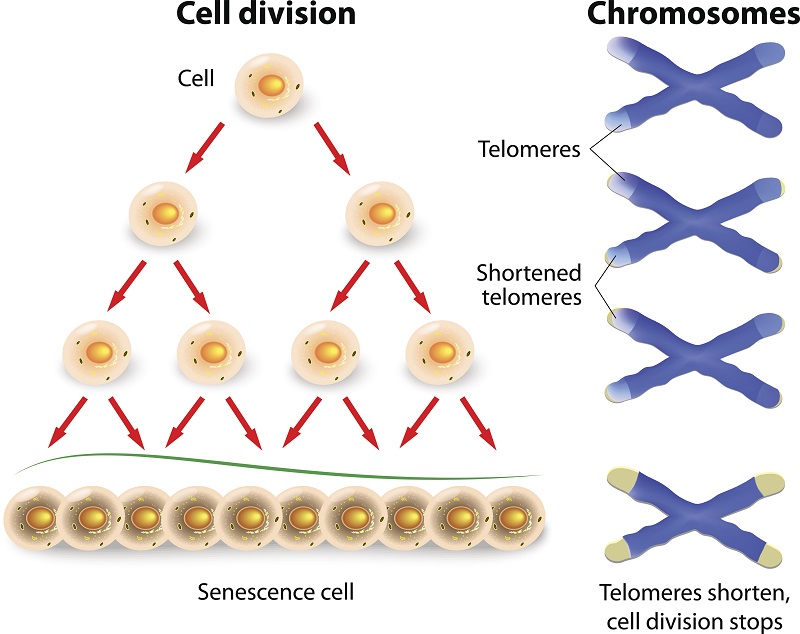
Although telomere shortening occurs naturally with each cell division, exposure to harmful environmental factors, such as free radicals, may accelerate telomere shortening. To counter
telomere shortening, some cells use the enzyme telomerase to maintain telomere ends. Ordinary cells don’t use telomerase and only human stem cells, reproductive cells, and cancer cells use this enzyme.
Because telomeres grow shorter as we age, scientists suggest using telomere length as a way to measure accelerated aging in individuals, a condition where a person’s biological age is older than their chronological age. Using telomere length as a marker of biologic age is an attractive idea, however, is plagued by many sources of variance which limit its reliability, say Aviv and Shay.
Accuracy of Telomere Length Studies
In the study, authors Aviv and Shay (A+S) convincingly argue that most epidemiological studies of telomere length are grossly inaccurate, for several reasons. The majority of the telomere length studies were performed only on adults, omitting children. The studies were conducted mostly on Europeans, excluding, for the most part, the other races who outnumber them. Lastly, say A+S, the studies were too small to overcome measurement errors.
Problems with Telomere Length Studies
According to Aviv and Shay, the overwhelming majority of epidemiological studies were performed on adults, and the vast variation in telomere length is observed before adulthood. Moreover, most genome-wide association studies (GWAS) of telomere length were performed on Europeans. These studies that omitted non-European populations failed to capture the role of the interaction between genes and environment in setting the optimal telomere length and its potential role in longevity and the chronic diseases of aging.
Lastly, the studies were too small to overcome measurement errors. In fact, the vast inter-individual variation of telomere length throughout the human lifespan “demands large sample sizes to detect the influence of specific factors.” The authors add that the measurements of telomere length “display a high measurement error in the majority of epidemiological studies.” The authors point out that most of these studies failed to increase their sample size to offset these high measurement errors, adding
“While high measurement errors can be offset by increasing sample size, most of these studies have disregarded power considerations indicating that hundreds, if not thousands, of subjects might be needed to obtain reliable LTL [leukocyte telomere length] results.”
The authors conclude that most epidemiological studies of telomere length are inaccurate, saying
“Lack of a systematic attention to LTL measurement error and power limitations explain in part inconsistent findings of association of LTL with a host of traits, including longevity in the elderly, sex and race. It is essential, therefore, that we step back and have a second look at major studies that used flawed design and sub-optimal TL measurements to miss important connections between TL and biological parameters that do exist and detect associations with traits and diseases where none exist.”
Telomere Length as Biomarker of Aging
Telomeres are often considered as biomarkers of aging and people with short telomeres are considered biologically older than their peers because their clock is ticking at a faster pace. Researchers have found that psychological stress, as well as free radical damage, accelerate telomere shortening and studies link telomere shortening with chronologic aging. Telomere damage is a type of macromolecular damage and too much of it induces cellular senescence and cell death. Researchers don’t know if telomere shortening is a symptom of accelerated aging or a cause of it.
Geroscientists traditionally regard telomere length as a biomarker of human aging, however, Aviv and Shay say it is a poor indicator of chronological age. Telomere length has also fueled the field of telomere epidemiology to identify people whose biological age is out of step with their chronological one.
The vast majority of studies that measure telomere length of only the white blood cells called leukocytes, which are far easier to measure. In other words, the telomere length of leukocytes is used as a proxy for the overall telomere length of the other cells in the body. In fact, the terms telomere length is a stand-in for “leukocyte telomere length” throughout the rest of this report.
Telomere Length Varies At Birth
Aviv and Shay are confident that telomere length is highly inheritable, and telomeres are longer in females than males. Moreover, telomeres are longer in individuals of African ancestry than those of European descent. Interestingly, A+S say that telomere length is also longer in offspring conceived by older men.
The Heritability Effect on Telomere Length
The environment plays a role in fashioning telomere length by modifying its pace of shortening say Aviv and Shay. For instance, when a growing fetus is exposed to air pollution and cigarette smoke, it results in shorter telomeres. However, a more significant component of telomere length is inherited. For a given age, approximately 60% of the variation between individuals in telomere length is inherited. Moreover, it seems, that our genetic code has its hands on the throttle that governs the pace at which our telomeres shorten. We also inherit the rate at which our telomeres grow shorter, and 30% of our age-dependent attrition in telomere length is inherited, as A+S say
“The environment (in utero and during extra-uterine life) might play a role in fashioning LTL by modifying its pace of age-dependent shortening. For instance, exposure to air pollutants in utero [inside the womb] and cigarette smoke are associated with short LTL. However, a major component of LTL is constitutive. For a given age, approximately 60% of the inter-individual variation in LTL and 30% of its age-dependent attrition are heritable.”
Telomere Length Determines Maximum Human Lifespan
Aviv and Shay say that telomere length could impose a maximum lifespan on humans.
All somatic cells can divide a limited number of times before the stop, a phenomenon known as the Hayflick Limit. Cells hit this limit when their telomeres reach a critically short length. The average cell will divide between 50-70 times before cell death, but varies depending on cell type. Many cells are nowhere near the Hayflick Limit in older adults.
Aviv and Shay say despite the inaccuracies in using telomere length as a biomarker of aging, that telomeres could impose a ceiling on the lifespan of humans. Aviv and Shay say that the balance of tradeoffs between long and short telomeres
“is of particular relevance given that in advanced societies longevity has almost doubled in the last two centuries and in light of an ongoing debate that has left unsettled whether there is a natural limit to the human lifespan. Whether or not TL [telomere length] dynamics plays an active role in the ageing process itself, in principle, TL could impose a ceiling on the lifespan of many humans.”
Bottom Line
Given the wide range of fluctuation in inherited telomere length, combined with measurement inaccuracies in epidemiological studies, telomere length is a poor biomarker of aging. A+S suggest that to effectively use telomere length as a biomarker of aging for a particular individual, one would have to obtain a baseline reading at birth.
These inaccuracies also cast doubts on other studies which use telomere length as a biomarker of the chronic diseases of aging. Future studies with better accuracy will be required to better use telomere length as a biomarker of chronic disease.
This Series on Telomere Length Continues
Part 2 on the role of telomere length in cancer is here. Part 3 on the role of telomere length in chronic diseases is here.
This article is featured in the best of telomere reports series.
Show Us Some Love
- Share this post on social media and help us spread the word– It only takes one click on any of the social media links on this page.
- Follow us on social media – Google+ or Reddit
- Sign up for our email list – We use your email to notify you of new articles. We will not send you spam, and we will not share your email address. You can cancel at any time.
- Tell us what you think – Scroll down to enter your comments below.
References
Cover photo credit: Shutterstock.
Abraham Aviv, Jerry W. Shay. “Reflections on telomere dynamics and ageing-related diseases in humans.” Philosophical Transactions of The Royal Society B Biological Sciences, Published 15 January 2018.DOI: 10.1098/rstb.2016.0436. Link to Article.
Disclaimer
Diagnosis, Treatment, and Advice: This article is intended for educational and informational purposes only and is not a substitute for qualified, professional medical advice. The information and opinions provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. Consult a qualified and licensed physician for the diagnosis and treatment of any and all medical conditions. Experimental therapies carry a much higher risk than FDA-approved ones. Call 911, or an equivalent emergency hotline number, for all medical emergencies. As well, consult a licensed, qualified physician before changing your diet, supplement or exercise programs.
Photos, Endorsements, & External Links: This article is not intended to endorse organizations, companies, or their products. Links to external websites, mention or depiction of company names or brands, are intended for illustration only and do not constitute endorsements.
Hi, Brady:
The problem with the diagram in this post is that it is too simplistic. It ignores what we know about the stem cell basis of metazoan tissue development and maintenance, mammals in particular. Because long-lived stem cells are responsible for the asymmetric renewal of relatively short-lived mature tissue cells, what really matters for telomeres in aging mechanisms is what happens to their lengths in tissue stem cells. Now, since tissue stem cells have been shown to undergo non-random, immortal DNA strand co-segregation both in vitro and in vivo, there is a strong prediction (first noted by Christopher Potten) that their telomeres either do not shorten, even in the absence of telomerase, or they shorten at a much lower rate than the rate expected for cells dividing as depicted in the diagram with random sister chromatid segregation.
Unless our aging hypotheses are more comprehensive of such complexities, we are not going to discover how to delay or reverse human aging processes.
Tell Calico to call Asymmetrex.
Kind regards,
James
Director at Asymmetrex
Hi – it’s a stock photo, so it is simplified.