Can We Rejuvenate Our Bodies Using Telomerase to Lengthen Telomeres?
Summary: Telomerase replenishes telomeres, extending the lifespan of our cells. While much work remains to be done, researchers are hopeful that telomerase enhancing therapy will rejuvenate our organs and extend our lives. This article first appeared on LongevityFacts.com, follow us on Google+ | Facebook | Reddit. Author: Brady Hartman.
The enzyme telomerase replenishes telomeres, providing cells with an extended lifespan. Geroscientists hope that adding extra telomerase will protect the length of our telomeres and breathe new life into old tissue.
One would think that we could supplement with telomerase and live longer, but the story is decidedly more complicated. Some scientists believe that too much telomerase stokes the growth of cancer.
Looking very much like the plastic tips on the ends of shoelaces, telomeres are the protective caps on the ends of all human chromosomes. The word telomere is an amalgam of the Greek words ‘telos’ meaning end and the word ‘meros’ meaning part. Doctors already know that that short telomeres cause the diseases of old age and geroscientists suspect that telomeres cause the aging process itself. Fortunately, an enzyme called telomerase helps replenish telomeres.
Telomeres are repeated stretches of DNA that maintain the stability of our chromosomes and protect our genetic data. Unfortunately, when the telomeres wear away – due to aging or environmental stresses – our DNA becomes vulnerable to degradation. Shorter telomeres cause genomic instability and may be a risk factor for developing cancer. Telomeres also act as the biological clock of our cells. In healthy aging, each time a cell divides, its telomeres get shorter, until the cell either undergoes a form of cell death called apoptosis or becomes senescent.
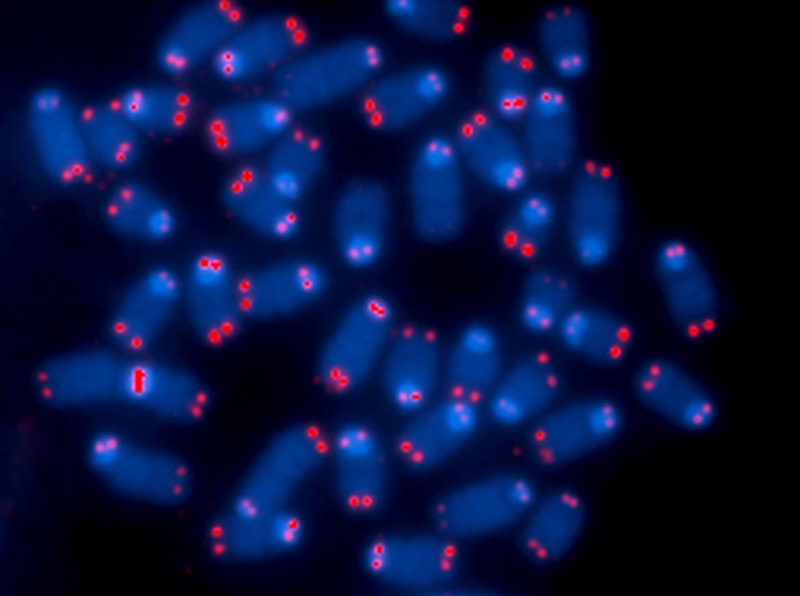
Telomerase Rejuvenates Telomeres
In the 1930s, researchers Hermann Muller and Barbara McClintock noticed that telomeres – the tiny caps on the ends of our chromosomes – had unique protective properties. However, in the intervening years, scientists did not know how telomeres functioned.
Fast forward to the 1970s, a decade in which researcher Elizabeth Blackburn identified the first telomere sequence at Yale University. Since then, the telomere research field has exploded, with Blackburn and other scientists transforming our understanding of how our cells use telomeres and telomerase in life and death. In 2009, Blackburn, together with Jack Szostak and Carol Greider received the Nobel Prize for discovering how telomeres protect the ends of our chromosomes and how telomerase replenishes telomeres.
Why Do We Need Telomeres Anyway?
Some cells experience wear and tear and need to be frequently replaced, such as skin cells and the cells lining our gut. These cells need telomeres to divide, and without these protective caps, they are unable to reproduce.
As well, telomeres protect the chromosomes by preventing the loss of genetic material during cell division. Telomeres protect the ends of chromosomes from fraying and breaking. Broken chromosomes cause all sorts of trouble and trigger ill health.
Telomere Length and Lifespan
The great paradox is that telomere length is indirectly proportional to lifespan. Conversely, as an organism ages, it’s average telomere length decreases. Said in another way, the fact of the matter is that long telomeres are associated with short lifespans and short telomeres with shorter ones. It doesn’t make intuitive sense, but those are the facts.
This paradox challenges the notion that longer telomeres are better. For example, mice have much longer telomeres than humans but live only two years on average.
The bottom line is that if you believe in reincarnation and want to come back as a long-lived animal, then pick a species with short telomeres, such as the bowhead whale. Once you’ve selected your new body, then you will want to keep your telomeres as long as possible, so you can keep swimming the seven seas. The remaining barrier to a long life will be the occasional assaults by Japanese whalers.
Telomeres – Cellular Clock or Time Bomb?
Telomere shortening is a significant factor limiting cell division. Some researchers have likened telomeres to a biological, genetic clock. Other scientists describe the telomere as ‘the fuse on a time bomb.’ This fuse becomes shorter with each cell division until it sets off a cellular time bomb that wreaks havoc on the cell.
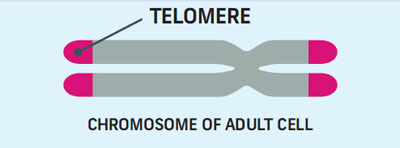
Telomeres act as a clock to keep the cell from dividing too much. Every time a cell divides, the telomeres get shorter. Once a cell has replicated about 50 times, it’s time for it to self-destruct. To avoid this fate, immortal cells use telomerase.
Telomerase Rebuilds Telomeres
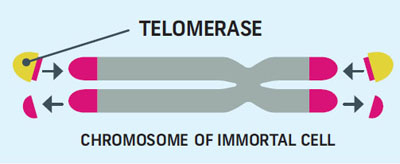
Many different types of proteins are involved in telomere up-keep, the most important one is telomerase. As a cell divides, it makes two copies of its chromosomes. This process gets messy and can tatter the telomeres. Telomerase rebuilds the shortened ends.
Our bodies have two types of cells: stem cells which are immortal, and regular cells, called somatic cells which have a limited lifespan. Somatic cells don’t use telomerase, while immortal cells do.
Telomeres With Telomerase

Stem cells and embryo cells and are involved in growth and regeneration and need to be immortal to do their jobs. They don’t like being limited, so they add back their telomere units using telomerase. Telomerase keeps their telomere length the same despite repeated cell divisions.
Unfortunately, cancer cells use telomerase as well. They also keep their telomere length constant and remain immortal, until they kill their host.
Telomeres Without Telomerase
Ordinary cells are prohibited from using telomerase, and their telomeres shorten with each division until they die. When all a cell has divided about 50 times and all the telomeres have been removed, the cell usually self-destructs, in a process call apoptosis.
How Telomeres Work
Like the rest of the material in our chromosomes, telomeres are composed of double strands of DNA. Human telomeres form t-loops—special folded structures where the single-stranded tail of the telomere is tucked into the internal double-stranded part. T-loops play an essential role in protecting the chromosome.
Research published in the Proceedings of the National Academy of Sciences suggests that when the T-loop unravels, it triggers the end of a cell’s reproductive life. The end of a cell’s life, known as cell death, can be brought about by DNA damage or due to short telomeres.
Researchers, Alexei Olovnikov and James D. Watson, discovered that DNA replication machinery cannot completely copy chromosome ends. Dr. Watson deemed this phenomenon the end replication problem. Each time a cell divides, it doesn’t completely replicate the chromosome ends making the telomeres a bit shorter. Eventually, telomeres become critically short, preventing further cell division. This cellular aging phenomenon is known as replicative senescence or the Hayflick limit, named after Leonard Hayflick who discovered the phenomenon in the early 60’s.
Once a cell has whittled down its telomeres to the nub, it has two choices: suicide or cellular senescence. Suicide via a form of cell death called apoptosis is the preferred route because senescent cells produce inflammation and wreak havoc throughout the body. Senescent cells are both universal and troublesome to us in our old age. So problematic, in fact, that scientists have developed compounds called senolytics that magically remove them from the body.
As a recent research-based report shows, some senolytics are better at removing senescent cells than others.
When Telomeres Go Haywire
Sometimes telomeres have flaws in their capping function, which leads to all sorts of problems in the body. Researchers suspect that these defects are related to the end loops unraveling. A telomere can lose its capping function if it becomes too short or is deleted entirely, or if a telomere protein is mutated or missing altogether.
Our cells are sensitive to the presence of broken chromosomes and treat them as a severe form of DNA damage. Whenever a cell detects uncapped chromosomes, it perceives them as broken DNA ends. Most of the time, broken DNA ends spark DNA repair mechanisms. Otherwise, the damaged DNA could cause ill health.
In addition to stimulating DNA repair processes, uncapped telomeres can trigger other cellular responses. Unrepaired DNA ends can trigger cellular growth arrest, preventing future cell division until the fractured ends are repaired. Broken DNA ends can also trigger cellular suicide, in a process called apoptosis. Because short telomeres are more common in older cells, telomere capping problems may increase the development of cancer and the other diseases of old age.
Some cells, however, refuse to self-destruct and become the living dead of the cellular world, known as senescent cells. Senescent cells produce buckets of inflammatory compounds and cause a great deal of trouble throughout the body. In fact, geroscientists implicate age-related chronic inflammation, a phenomenon known as inflammaging as a significant source of aging. Fortunately, researchers are developing anti-aging senolytic drugs, compounds which rid the body of worn-out senescent cells.
Using Telomerase To Lengthen Telomeres
In a landmark experiment, a group of geroscientists showed that telomerase therapy extended the lifespan of mice, without causing cancer.
Researchers have hailed telomerase therapy as a way to help us live longer and healthier. Scientists can lengthen telomeres by stimulating the production of the enzyme telomerase. The theory is that longer telomeres will lead to longer, healthier lives.
In one experiment, researchers inserted telomerase into mice with the aid of a viral vector. Researchers the mice into two sets: adult mice and elderly mice, and treated them with telomerase. At the conclusion of the experiment, the researchers noticed that the telomerase-treated adult mice lived 24 percent longer while the older mice lived only 13 percent longer. Notably, the treated mice did not have the dreaded side effect of increased cancer.
While these results are promising, some level-headedness is in order. Mice are not humans and have longer telomeres. Furthermore, what works splendidly in animals often fails when tested in humans.
Other Ways To Lengthen Telomeres Besides Telomerase
Scientists have shown that adding telomerase to cells in a test tube allows them to reproduce indefinitely. However, telomerase isn’t the only way to lengthen telomeres. As a recent experiment show, RNA therapy gets the job done as well.
Consider children afflicted with progeria, a disease that ages children so rapidly that they die in their teens from the conditions of old age. The chromosomes of children with progeria have exceptionally short telomeres. Scientists believe that the disease of progeria causes rapid cell turnover. Their cells use up their ability to reproduce, which in turn may contribute to the premature aging of children with progeria.
In a report on RNA therapy published earlier this year, Dr. Cooke showed the novel technique to lengthen the telomeres of human cells in a test tube allowing them to live longer. Unfortunately, what works in a test tube usually doesn’t work for a living, breathing body.
Can We Lengthen Telomeres To Stop Aging?
Researchers believe that shortening of the telomeres leads to the aging of cells and of the whole organism. Scientists also think that the telomere mechanism evolved to prevent cells replicating infinitely. While telomerase makes cells immortal, it also makes cancer possible.
Telomeres shorten with age, producing senescent cells, a significant source of inflammation and the chronic diseases of aging. In theory, supplementing with compounds that stimulate additional telomerase could lengthen telomeres. In turn, this will prevent the degenerative processes that accompanying aging such as arthritis, heart disease, type 2 diabetes, and cancer.
Should We Lengthen Telomeres With Telomerase?
Geroscientists are divided on the answer. One side argues that using telomerase or other therapies to lengthen telomeres can practically eliminate the chronic diseases of aging, from type 2 diabetes to cancer. The other side urges cautions because cancer cells also use telomerase to make themselves immortal. This group believes that adding more telomerase to the mix will riddle our bodies with cancer.
Tissue engineers hope to overcome organ failure by infusing cells with telomerase. This is based on the knowledge that all cells have a gene which turns the production of telomerase. As the theory goes, because the aging cell has low telomerase levels, perhaps these engineers can turn on this gene to lengthen the lifespan of a cell, which in turn rejuvenates the organ.
In this process, a technician takes cells from a patient and then places them in a culture and modifying them with gene therapy that stimulates extra telomerase, after which a doctor puts them back in the patient.
Thus far, researchers have used seventeen different cell types have been used to engineer a total of twenty-two different kinds of tissues. This technology is substantially limited owing to the limited understanding of the complexity of aging cells.
Using a patient’s own cells to build new tissue has an inherent drawback – cells have a limited lifespan, especially those from older patients. A researcher can overcome this limitation if they first infuse the patient’s cells with a specific gene, called hTERT, that switches on the gene that produces telomerase. The extra telomerase then restores the length of the shortened telomeres.
Scientists propose a form of therapy that lengthens telomeres with hTERT. First, a technician obtains shortened telomeres from a patient and places them into a culture. The technician then restores the telomere length with an infusion of hTERT. A technician then reintroduces the cells back into the patient’s organs to restore tissue function. Tissue engineers propose using this novel technique in many different tissue engineering applications, such as in arterial bypass grafting in elderly cardiac patients.
Does Telomerase Cause Cancer?
A Duke University study suggests that telomerase by itself is not capable of inducing cancer.
In research carried out on cells in test tubes, the scientists noticed that overexpression of telomerase failed to produce malignant changes. The Duke study researchers could not find evidence of tumor formation of either the hTERT cells or the control cells.
As well another group of researchers reported that long-term effect of telomerase does not produce cancer in cultured cells. A team of scientists at the UT Southwestern Medical Center immortalized human fibroblast cells with telomerase. The UT researchers reported that, over the long term, these telomerase-treated cells did not show the changes associated with cancer.
The UT scientists also noted that the age-related changes to cells did not reverse, even after the transplantation of telomerase modified cells. In fact, the researchers noticed that collagen synthesis decreased and reduced the strength of the engineered tissue.
Unfortunately, what works splendidly in a test tube usually doesn’t work as well in a living body.
While cell therapy with hTERT holds tremendous promise, researchers are still unsure about the long-term risks. For example, might hTERT-modified cells be more dangerous if the mutational changes activate cancer genes? Doctors need to balance the potential risk of hTERT gene therapy with its ability to save lives.
Before these this technology can be used, researchers need to develop a better understanding of how these newly engineered tissues develop and age.
The Bottom Line on Telomerase and Telomere Therapy
Researchers are intrigued by the observation that as people’s telomeres get shorter, their risk of death and the chronic diseases of aging rises. So one would think that any treatment that rebuilds or lengthens telomeres would be an instant blockbuster. However, scientists fear that enhancing telomerase may also lead to cancer. While animal and test tube experiments are not conclusive, the mouse, Duke and UT experiments cited earlier show that this fear is unfounded for the most part.
While telomere therapy with telomerase offers tremendous promise in clinical medicine, it is hampered by remaining questions of safety. Scientists must answer questions such as what is the risk of cancer? Moreover, how likely is this therapy to trigger abnormal tissue growth over the long term? Research into telomeres and telomerase is still in its early phases. Geroscientists have learned a great deal, and the emerging technology offers enormous potential for controlling the diseases of old age.
Telomerase therapy has the potential to be used in rejuvenation and regeneration applications. The most promising use of this technology is in the field of regenerative medicine.
Related Article: Learn more about the promising potential of gene therapy to rejuvenate an aging body.
Help Us Spread the Word
Please share this report and help us spread the word. All it takes is one simple click on any of the social media links shown on this page.
References
P. Scaffidi, L. Gordon, T. Misteli. The Cell Nucleus and Aging: Tantalizing Clues and Hopeful Promises. PLoS Biology Vol. 3/11/2005, e395 doi:10.1371/journal.pbio.0030395. Available Online.
De Jesus, E. Vera, K. Schneeberger, AM Tejera, E. Ayuso, F. Bosch, & MA Blasco. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. August 2012, EMBO Molecular Medicine. doi:10.1002/emmm.201200245. Available Online.
Klinger, Rebecca Y. et al. “Relevance and Safety of Telomerase for Human Tissue Engineering.” Proceedings of the National Academy of Sciences of the United States of America 103.8 (2006): 2500–2505. PMC. Retrieved from the Web. 13 Oct. 2017. Available Online.
Carmela P. Morales, Shawn E. Holt, Michel Ouellette, Kiran J. Kaur, Ying Yan, Kathleen S. Wilson, Michael A. White, Woodring E. Wright & Jerry W. Shay. Absence of cancer−associated changes in human fibroblasts immortalized with telomerase. Nature Genetics 21, 115 – 118 (1999) doi:10.1038/5063. Available Online.
Disclaimer
Diagnosis, Advice, and Treatment: This article is intended for informational and educational purposes only and is not a substitute for professional medical advice. Experimental treatments such as hTERT, RNA Therapy and other therapies which increase telomerase carry a much higher risk than FDA-approved ones. The information provided in this report should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. Consult a licensed physician for the diagnosis and treatment of any and all medical conditions. Call 911, or the equivalent emergency hotline number, for all medical emergencies. As well, consult a licensed physician before changing your diet, supplement or exercise programs. Photos, External Links & Endorsements: This article is not intended to endorse companies, organizations or products. Links to external websites, depiction/mention of company names or brands, are intended only for illustration and do not constitute endorsements.

Hello, Brady:
The whole of the telomere story is a wonderful narrative of biology and scientific discovery. It is one of the deductive marvels of molecular and cellular biology. The chromosome end problem and the prediction of the existence and necessity of a “telomerase” activity in dividing cells emerged from Meselsohn and Stahl’s demonstration that DNA replication was semi-conservative as intuited from the awe of Watson and Crick’s base complementary double helix structure for DNA. The proof of the existence of telomerase by classical biochemistry led to another set of Nobel prizes. What a grand story in biology, and great for teaching new students in the life sciences how deductive reasoning and experimentation can integrate to define amazingly real features of living beings.
But after that, the story is more uncertain than the current deductive reasoning about the role of chromosome ends and telomerase in processes like cancer and aging. In the deductive process, one must pay close attention to hobgoblins that don’t fit into the general theory…and don’t forget about them as comfort with the conventional thinking grows. Okay, so below is a list of hobgoblins that stay on my mind to call into question the currently reigning concepts that reduced or lost telomerase contributes to human aging and activation of telomerase is an important factor in the initiation of cancers. Let me say here, though, that the experimental data are actually quite good to support the idea that mutations that increase or maintain telomerase activity can promote the progression of tumors. It’s the role of telomerase in the initiation of cancer cells and aging that I’m calling into question.
1. Let’s get back to the early experiments that were the basis for concluding that loss of telomerase activity is responsible for the senescence of primary mammalian cell cultures. My research teams’ work has suggested an alternative explanation, namely the serial dilution of asymmetrically self-renewing tissue stem cells by their progeny cells that have a limited division potential. Here’s the hobgoblin. The time at which a primary cell culture attains senescence depends greatly on how frequently and how much it is diluted during serial passage. No one has explained this well known property in terms of the telomerase hypothesis. It has been mostly ignored. Yet, it is easily explained by the dilution rate of asymmetrically self-renewing tissue stem cells, because stem cell asymmetric self-renewal has been shown to be cell density dependent.
2. The key tissue cell type for the proposed effects of telomere metabolism on aging and cancer is the asymmetrically self-renewing tissue stem cell. There are two hobgoblins here that are continuously overlooked. First because of the formidable problem of specific identification of tissue stem cells, their actual telomerase expression state remains largely imagined and actually unknown. Beware reports claiming to have made such crucial determinations. Always look carefully at either the purity of the “stem cells” examined and the basis for their designation as tissue stem cells. Second, given the existence of non-random immortal DNA strand co-segregation in asymmetrically self-renewing tissue stem cells, there is NO chromosome end problem! Telomerase becomes irrelevant for preventing DNA replication induced shortening, because new and old DNA strands are not randomly retained by the stem cells. Of course, telomerase might still join other chromosome end associated complexes to insure proper chromosome function, but this distinction could be very important to make for an accurate view of what is most responsible for initiation of aging and cancer.
Best,
James at Asymmetrex
Thank you so much, Dr. Sherley for adding that piece. You have shown how researchers are still stumbling in the dark on some aspects.