New Liquid Biopsy Tests Aim to Prevent Cancer Mortality
New liquid biopsy tests hold promise to prevent most cancer deaths with a simple, inexpensive blood draw. [This article first appeared on LongevityFacts. Author: Brady Hartman. ]
A simple blood test that tells if you have a tumor and where it is in your body is a lot closer to reality and may cost only $500. Johns Hopkins University (JHU) just announced a test earlier this year that detects eight common types of cancer. It may prove inexpensive enough to be prescribed during a routine physical.
The JHU test is not the only early warning test for cancer. In fact, several organizations are developing new low-cost ways of detecting cancer early, creating the closest thing we have to a silver bullet cure against the disease.
The new crop of liquid biopsy tests is a significant step toward one of the hottest goals in cancer: blood tests that can detect tumors early before a person feels sick or notices a lump. It’s useful because early-stage cancer that hasn’t spread can often be cured.
JHU’s New Liquid Biopsy
As reported in January in the journal Science, JHU’s new test, called CancerSeek detected an existing tumor about 70% of the time across eight common cancer types in 1005 patients whose tumors had not yet spread. Making it among the best performances yet for a universal blood-based cancer test. JHU’s new liquid biopsy also narrowed down the type of cancer, which previous universal blood-based cancer tests have been unable to do. Although the test, isn’t commercially available yet, it will be used to screen 50,000 women with no history of cancer at the Geisinger Health System (GHS) in Pennsylvania.
Detection rates varied depending on the type — lower for breast cancers but higher for pancreatic, ovarian, and liver tumors. In many cases, the blood biopsy narrowed the possible origin of the tumor to one or two locations, such as the lung or colon. The blood test gave only seven false alarms when tested on 812 others without cancer.
Liquid biopsies are based on the idea that genetic mutations drive the growth of cancer cells, and as a tumor develops, it sheds its telltale signature into the bloodstream. The ideal diagnostic is easy to sample, easy to detect and has a low rate of false positives. CancerSEEK detects the presence of these genetic mutations as well as the levels of 8 proteins. The JHU team used an AI technique called machine learning to train the CancerSEEK algorithm to detect signs of cancer.
In blood samples from patients with eight types of tumors, the test detected between 33% and 98% of cases, depending on the type of cancer. The average tumor had progressed to stage II in the patients tested. Stage II cancers accounted for 49% of the patients, with 20% at stage I and 31% at stage III of the disease.
As for the entire sample, which had progressed to stage II on average, CancerSEEK detected 69% or higher of ovarian, stomach, pancreatic, and esophageal cancers, all types that are difficult to detect early. The test fell short of the mark, detecting breast, colorectal and lung cancers.
The test rarely found a tumor that wasn’t there, and less than 1%, of the healthy controls, tested positive. The JHU team estimates the cost of their liquid biopsy at less than $500 per sample.
One of the most important attributes of a screening test is the ability to detect tumors at relatively early stages. CancerSEEK could detect 73% of Stage 2 cancers and 78% of those at Stage 3. Unfortunately, the test could only identify 43% of stage 1 cancers. However, the test varied in sensitivity depending on the type of cancer, detecting anywhere from 100% of stage 1 liver cancers to 20% of stage 1 esophageal cancers.
When weighted for actual incidence in the U.S., the authors estimate that CancerSEEK could detect 55% of the eight cancer types. Importantly, the test could detect tumors in the ovaries, liver, stomach, pancreas, and esophagus. Cancers for which there are no early screening tests available for average-risk individuals.
Despite its shortcomings, JHU’s liquid biopsy could save hundreds of thousands of lives. As the authors say in their study,
“The eight cancer types studied here account for 360,000 (60%) of the estimated cancer deaths in the U.S. in 2017 and their earlier detection could conceivably reduce deaths from these diseases. To actually establish the clinical utility of CancerSEEK and to demonstrate that it can save lives, prospective studies of all incident cancer types in a large population will be required.”
To that end, the Johns Hopkins team thinks CancerSEEK is ready for testing as a screening tool. The Geisinger Health System in Pennsylvania has already begun to use JHU’s liquid biopsy system on blood samples from female volunteers between ages 65 and 75 who have never had cancer. The planned 5-year, $50 million study of up to 50,000 women is being funded by The Marcus Foundation, a private philanthropic group.
Liquid Biopsy Gold Rush
Other firms are detecting cancer in the blood and breath based on the telltale signatures that tumors leave behind, including DNA mutations, protein expression, or different types of RNA. A startup called CellMax has developed a device to detect cancer from a simple blood test. Another firm called Owlstone is working on an invention that detects cancer on the breath.
Once a backwater of cancer research, the field of cancer detection is rapidly emerging as a way to slash cancer deaths in our lifetimes. With the new tests, you don’t need to have any symptoms, just a bit of blood (or breath) and a low-cost testing system. Today, cancer detection happens mainly when symptoms appear. That is after the cancer has spread to other organs when it is too late. For nearly all tumors, early detection and treatment are the best shot we have at curing cancer.
Early Detection Saves Lives
As it stands now, one out of three Americans who get cancer will die of the disease because the tumors are usually detected too late. New cancer early-detection technology, such as the liquid biopsy, could lead to the day when people are routinely screened to catch tumors before they cause symptoms when chances are best for a cure.
Early cancer detection has already proven its merit in several types of cancer. For example, the National Cancer Institute (NCI) reports that, as of March 2015, early detection and treatment of breast, colon, lung and cervical cancers are already saving countless lives. Until recently, diagnostics have been sorely lacking for many other forms of cancer, such as lung, pancreatic, and liver cancers, which are usually fatal because they are diagnosed too late.
New ways of addressing cancer such as precision cancer treatments, targeted cancer therapies, preventative cancer vaccines and cancer immunotherapies such as CAR T-cell therapy hold a great deal of promise. However, these treatments are incredibly expensive, and they cover a fraction of the varieties of cancer.
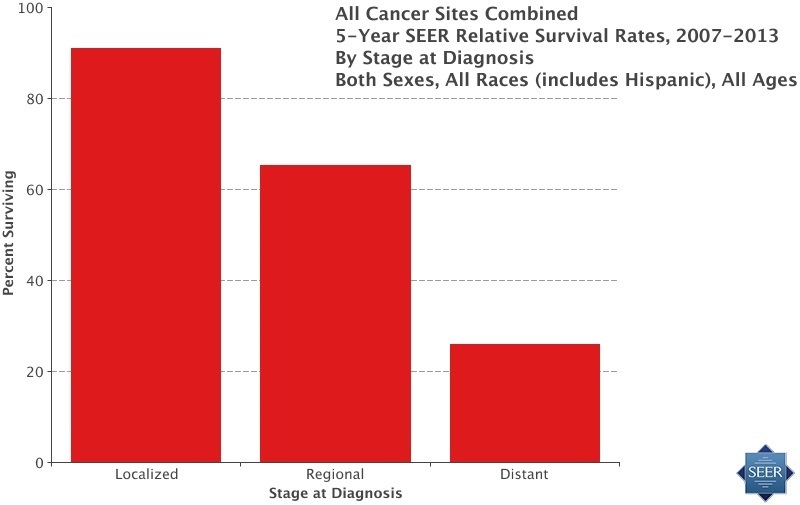
Existing cancer treatments work best on cancers detected at its early stage. According to the latest statistics from the NCI’s SEER database, treating cancer in the earliest stages yields about a 90% survival rate on average, when using existing standard treatments. Universal screening and detection of tumors could conceivably prevent hundreds of thousands of cancer deaths in the US each year and ten times that number worldwide.
For example, take colon cancer, as with many other types of tumors, there are often few symptoms until the cancer is at an advanced stage. According to the latest SEER statistics, 91% of patients survive when colorectal cancer is detected early, in what is called the localized stage. A promising immunotherapy to treat aggressive colon cancer is in the development pipeline. However, as it stands today, only 14% survive, once the tumor has metastasized, reaching what is referred to as the distant stage.
CellMax Liquid Biopsy
Taiwanese firm CellMax Life is also developing a promising liquid biopsy which detects cancer at the earliest stages by looking for DNA and other molecules that tumors shed into the blood. In early trials, the test performed well at detecting cancers that currently lack screening tests.
The liquid biopsy identifies circulating tumor cells (CTCs) – cancer cells that detach from a primary tumor and flow through the bloodstream. CTCs have long been valuable in malignancy detection, but most technologies using CTCs are only able to detect late-stage cancer. The CellMax liquid biopsy system detects these tumor cells in the blood at the earliest stages by processing blood samples using a lipid-coated chip that mimics human tissue. In prior studies, this system was able to detect tiny numbers of the CTCs, even down to the level of one tumor cell per billion blood cells, found in colon polyps.
The fundamental mechanism of circulating tumor cell shedding, and its detection using liquid biopsy are the same across all solid tumors. Thus, this approach is expected to provide favorable results for other solid tumors such as breast, lung and prostate cancers.
The CellMax liquid biopsy seems to perform better than standard lab tests at detecting colorectal cancer, as well. In January 2018 the researchers presented a study at the ASCO conference which showed that the Cellmax test detected early-stage colorectal cancer and pre-cancer with high accuracy.
A new study showed that its circulating tumor cell blood test could detect colorectal cancer (CRC) at the early stage – and in many cases detect pre-cancerous growths with an accuracy superior to that of the fecal occult blood testing (FOBT), a standard test for CRC screening.
Testing the CellMax Liquid Biopsy
The researchers enrolled 620 adults who were either undergoing routine colonoscopies or had confirmed colorectal cancer. After a colonoscopy and biopsy, 438 of them were found to have either pre-cancerous growths called polyps or early to late-stage colorectal cancer. The remaining people had no signs of pre-cancerous polyps or CRC and were the comparison group.
The study results showed that the test’s sensitivity, or how well it detected known cancers, ranged from 77% for detection of CTCs in pre-cancerous growths to 87 percent for stage 1-4 tumors.
CellMax’ liquid biopsy is nowhere near ready for use yet; it needs to be validated in a more extensive study in a general population, rather than cancer patients. The researchers are planning more studies in Taiwan and the United States.
Breath Biopsy
Billy Boyle, the president of Owlstone Medical, a small biotech company based in Cambridge, England, has invented a novel device to detect cancer on the breath. The idea behind the invention is that the breath contains a wide variety of organic compounds that reflect what is going on in the body’s metabolism. Because cancers affect the metabolism, they should also change the pattern of compounds in the breath. Owlstone’s novel invention called the Breath Biopsy uses an ion-mobility spectrometer to weigh molecules by passing them through an oscillating electric field. The system is tiny, fitting on a chip the size of a coin as well as sensitive, identifying compounds at a level of a few parts per billion.
Owlstone’s technology platform is the subject of several current trials. For example, in July of last year, the firm kicked off the PAN Cancer trial to evaluate the use of Breath Biopsy system for the early detection of cancers of the brain, esophagus, bladder, breast, head, neck, kidney, pancreas and prostate. The trial plans to identify breath biomarkers to improve the early detection and diagnosis of multiple types of cancer, with the ultimate aim of speeding up diagnosis for significantly improved treatment results. The firm hopes that when it has identified the molecular fingerprints associated with particular tumors, it will be able to detect cancer earlier than other tests.
The Breath Biopsy platform is also the subject of another trial called LuCID, which is recruiting 4,000 patients across Europe to develop a test for the early detection of lung cancer. Another trial is attempting to detect early-stage colorectal cancer in 1,400 people, hoping to improve on existing screening methods.
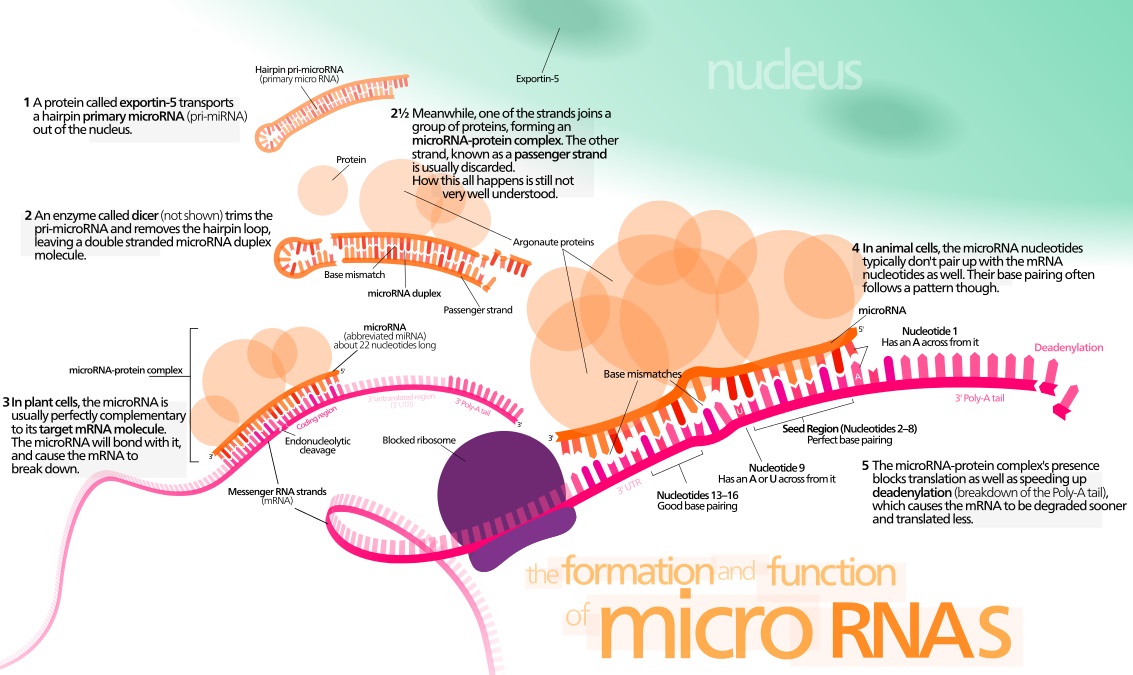
MicroRNAs as a Liquid Biopsy
Through his startup, Miroculus, Jorge Soto has built a device that he says reliably and accurately detects several types of cancer at the very early stages using a blood sample. Soto’s take on the liquid biopsy approach works by detecting a set of molecules shed by tumors that circulate freely in our blood called microRNAs.
MicroRNAs are class of RNAs that regulate various cellular processes, such as replication and differentiation. They make for an attractive target in a liquid biopsy because they are small, stable, and found in the blood.
All of the cells in our body use proteins to accomplish their jobs. However, cancer cells operate differently from normal cells and use a different set of proteins. MicroRNAs are small molecules that specify the types of protein to manufacture, by regulating gene expression. Unlike DNA, which is fixed, microRNAs can vary depending on the conditions at the particular moment.
While no two cancers are the same, there are patterns at the microRNA level. Several studies show that abnormal manifestations of microRNA levels create unique, specific patterns for each type of cancer at the early and subsequent stages of the disease, making microRNAs a highly sensitive biomarker.
Soto’s firm has built up a database that correlates microRNA patterns with the presence of a specific type of tumor. They then take a patient’s blood sample and compare it with the existing database, and using a computer processor to match it to, pancreatic cancer for example.
The entire solution costs at least 50 times less than currently available methods, according to Soto. So far, Miroculus claims they have been able to successfully identify the microRNA patterns of lung, pancreatic, liver, and breast cancers.
However, the use of microRNAs for cancer detection is controversial. An ideal biomarker is one that is present when the disease is present, and absent when it is not. MicroRNAs, on the other hand, are diagnostic in their concentration. For example, a particular microRNA is typically at or below a certain level and exceeds that threshold if an individual has a specific type of cancer. To be proven reliable, all other factors that could lead to a significant change in concentration without the disease present need to be eliminated, such as medications, diet, or exercise. Moreover, scientists have found it challenging to compare microRNA concentrations using different platforms, or even the same platform with different vendors’ systems. As a consequence, only a handful of microRNA-based products have been approved for cancer diagnostics.
The Miroculus system profiles microRNA and uses predictive modeling to narrow down the number of microRNAs associated with a particular disease. The company then validates this research using clinical studies. Miroculus has just completed an extensive clinical study on gastric cancer and is about complete a second one. The startup plans on publishing its findings and submitting the data to the FDA for evaluation shortly.
Liquid Biopsy Shortcomings
Early diagnosis of cancer has been peppered by shortcomings, most notably with prostate cancers. Some worry that these new liquid biopsies will detect small tumors that would never grow large enough to cause problems yet will be treated anyway, at unnecessary risk, cost and anxiety to the patient. The issue is not overdiagnosis, but overtreatment. In any case, it will take time to figure out whether widespread screening of healthy people with a liquid biopsy can reduce cancer deaths without harming.
No Liquid Biopsy to Rule Them All
We shouldn’t expect one brand of liquid biopsy to be the ‘one ring that rules them all.’ Cancer is not a single disease but a hundred individual diseases. Doctors use a multitude of tests to diagnose cancer, choosing the test best suited to the patient and the suspected type of tumor.
It remains to be seen whether the JHU liquid biopsy, the Breath Biopsy, the CellMax liquid biopsy, or the Miroculus system will prove clinically useful. Only clinical trials will provide the answer.
The point is that, with so many irons in the fire, one of them is bound to strike hot. If only one of these new liquid biopsies lives up to its promise in clinical trials, doctors will have a new suite of tools with which to diagnose cancer at its early stages.
Liquid Biopsy Takeaways
The day is fast approaching when cancer is routinely diagnosed at the very early stages and easily treated. Doctors will no longer be using decades-old ways to detect the disease. Because of these and other breakthroughs the way we see cancer will radically change.
Related: Vitamin D linked to reduced cancer rates by 20% in a new study.
Related: Viagra may reduce the risk of colorectal cancer, say scientists.
Like this Article?
- Help us spread the word – Click on any of the social media links to share this article.
- Follow us on social media – Google+ or Reddit
Cover Photo: © 2009 Andrei Tchernov / Getty Images.
Disclaimer
Diagnosis, Treatment, and Advice: This article is intended for informational and educational purposes only and is not a substitute for qualified, professional medical advice. The opinions and information stated in this article should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. Consult a qualified and licensed physician for the diagnosis and treatment of any and all medical conditions. Experimental liquid biopsy tests carry a much higher risk than FDA-approved ones. Dial 9-1-1, or an equivalent emergency hotline number, for all medical emergencies. As well, consult a licensed, qualified physician before changing your diet, supplement or exercise programs.
Photos, Endorsements, & External Links: This article is not intended to endorse organizations, companies, or their products. Links to external websites, mention or depiction of company names or brands, are intended for illustration only and do not constitute endorsements.
