Breakthroughs Set To Revolutionize Stem Cell Therapy
Summary: These breakthroughs in stem cell therapy could potentially rejuvenate our damaged organs with induced pluripotent stem cells (iPSCs). Moreover, biotech firms are rushing to bring organ-rejuvenating cell therapies to the marketplace. [This article first appeared on the LongevityFacts.com website. Author: Brady Hartman. ]
Recent advances in stem cell therapies could translate into effective treatments for intractable diseases.
Stem cells are the repairmen of our bodies. Unlike our ordinary cells, stem cells can divide without limit and create fresh copies of nearly any tissue type to repair damaged organs. While we have an abundance of these repairmen in our youth, we experience stem cell decline as we age.
The chronic diseases of aging such as AMD, heart disease and stroke, heart failure, orthopedic disorders, Alzheimer’s Disease, Parkinson’s disease, type 1 diabetes, and respiratory disease such as COPD affect millions of people. The underlying diseases permanently damage tissues and organs, turning these conditions into lifelong, chronic diseases. Stem cell therapy hopes to restore these damaged tissues and organs.
Here is an update of what the research organizations and the biotech industry are doing to help us reverse this stem cell decline by making fresh cells in the lab.
Origins of Stem Cell Therapy
It’s been more than ten years since Japanese researcher Shinya Yamanaka, M.D., Ph.D. developed the breakthrough technique to return any adult cell to its earliest stage of development and change it into different types of cells in the body. This technology called induced pluripotent stem cells (iPSCs) opens the door to rejuvenating many organs, including generating cardiac cells to restore damaged heart tissues, cartilage cell tissue to repair knees, and retinal cells to improve the vision of those with age-related macular degeneration (AMD) and other eye diseases.
Yamanaka’s Breakthrough
Dr. Yamanaka’s breakthrough came when he discovered four proteins or factors which force cells to revert to a state that resembles the embryonic stem cells found in a growing fetus. The discovery of these four Yamanaka Factors earned Dr. Shinya Yamanaka the 2012 Nobel prize along with Sir John Gurdon “for the discovery that mature cells can be reprogrammed to become pluripotent.”
Yamanaka factors induce matured adult cells taken from a patient’s body to revert to a younger state and form induced pluripotent stem cells. They are also called transcription factors because they turn DNA into RNA. The four Yamanaka factors are the proteins c-Myc, Klf4, Oct4, and Sox2.
iPSC Stem Cell Therapy
Using the Yamanaka factors, scientists can take adult cells that have already been differentiated – such as skin, hair or blood cells and turn into iPSCs. Moreover, once these adult cells have been regressed into iPSCs, they can be converted into any needed tissue.
For example, in what as known as stem cell therapy, a technician could extract the skin cells of a heart attack victim, manipulate them into iPSCs, and then change them back into progenitor heart stem cells. The technician could then transplant these progenitors back into the heart attack patient, where they would turn into muscle cells and restore the damaged heart, reversing heart disease.
Clone Wars
Despite its immense promise, doctors have been slow to adopt iPSCs for stem cell therapies. Physicians have been held back by concerns that induced pluripotent stem cells are prone to increased numbers of genetic mutations.
That all changed on February 6, 2017, when scientists at the National Human Genome Research Institute (NHGRI) announced they published a study which suggests that iPSC cells do not develop more mutations than cells that are duplicated by subcloning. Subcloning is a technique where single cells are cultured individually and then grown into a cell line. The technique is similar to the iPSC except the subcloned cells are not treated with the reprogramming factors which were thought to cause mutations. The researchers published their findings in the Proceedings of the National Academy of Sciences, one of the most respected medical journals. Pu Paul Liu, M.D., Ph.D., co-author of the study and deputy scientific director for the NHGRI’s Division of Intramural Research said
“This technology will eventually change how doctors treat diseases. These findings suggest that the question of safety shouldn’t impede research using iPSC.”
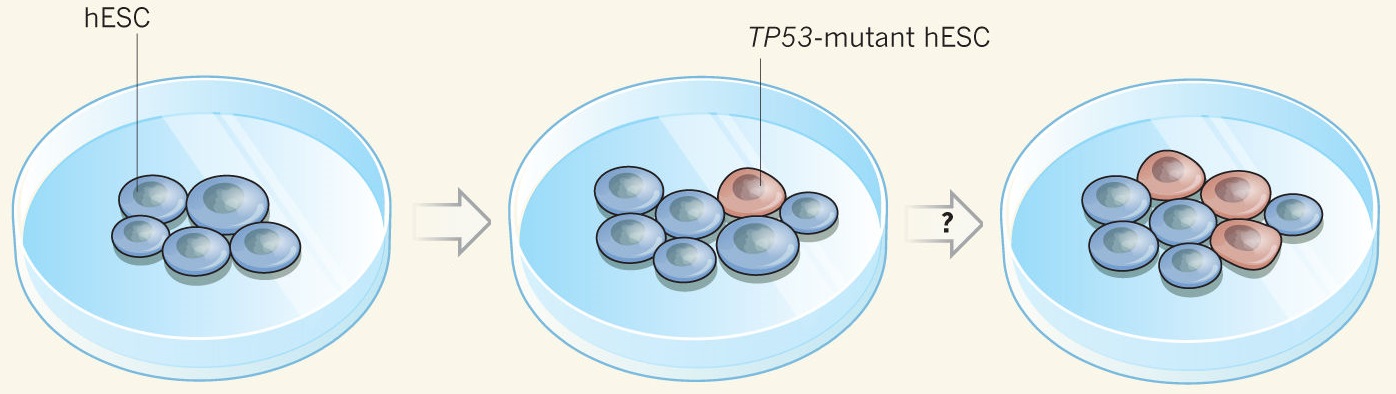
This was good news until Merkle and colleagues shocked the industry by publishing a study in the journal Nature in May which showed that some pluripotent cells developed potentially cancerous mutations. A companion article in the journal Nature said that Merkle’s team showed that pluripotent cells derived from human embryonic stem cells (hESCs) and cultured in a test tube are liable to develop mutations in the gene TP53, leading to cancer.
To sidestep this disastrous outcome, technicians have to perform the step of meticulous screening.
Growth in Stem Cell Therapy
Despite the concerns about mutations, scientists have rediscovered Yamanaka factors in the last year and are producing phenomenal results. Researchers and biotech companies are testing stem cell therapy on humans, fine-tuning the process and their successes are increasingly outnumbering their failures.
For example, Lung Disease News reported in August that researchers at University of North Carolina Health Care (UNCHC) are planning trials of stem cell therapy to treat respiratory diseases such as COPD, pulmonary fibrosis, and cystic fibrosis. The UNCHC researchers announced they had made great strides towards developing stem cell treatments for lung diseases and are discussing the initiation of clinical trials with regulatory authorities.
Early Failures with Stem Cell Therapy
There have been growing pains using Yamanaka factors to create iPSCs, a problem experienced by nearly all breakthrough technologies in their early years of development.
In the early experiments, scientists discovered that it is important to avoid prolonged use of Yamanaka factors and similar compounds to revert normal cells to a stem-like state. Nasty teratomas, or jumbles of teeth, tissues, and bone, as well as other tumors, can result if the treatment is more than temporary.
Embryonic cells are primed for rapid growth, and too much of a good thing has adverse consequences. Infusing Yamanaka factors as a whole-body treatment has had disastrous results in lab animals. Attempts to do this in 2013 and 2014 resulted in the deaths of all of the animals, either because of cancers or because the iPSCs lost their identities.
Moreover, the field of the stem cell therapy is still experiencing growing pains. As more and more labs adopt iPSCs, scientists struggle with consistency. For example, last year, Nature interviewed Jeanne Loring, a stem cell biologist at the Scripps Research Institute in La Jolla, California who said,
“The greatest challenge is to get everyone on the same page with quality control,” adding “There are still papers coming out where people have done something remarkable with one cell line, and it turns out nobody else can do it,” continuing “We’ve got all the technology. We just need to have people use it right.”
Stem Cell Therapy Successes
Based on the earlier experiments, scientists are tinkering with the formula in various ways to reduce mutations. Researchers are now using highly precise and targeted exposure to Yamanaka factors, and pulses – with some success.
For example, in September 2014 the journal Nature reported that Japanese scientists used stem cell therapy to improve the eyesight of an elderly woman by injecting retinal pigment epithelial cells derived from iPSCs. These were reprogrammed from cells taken from her own skin. The researchers at the Kobe City Medical Center General Hospital treated the woman with iPSCs to stabilize her age-related macular degeneration. Since the stem cell therapy, the brightness of her vision has improved and has remained stable for some years.
Improving on Yamanaka Factors
Scientists now aspire to create drugs that replicate and amplify the actions of these four Yamanaka factors, to perform stem cell therapy without the mutational side effects.
For example, a team of Chinese scientists led by Danwei Huangfu has investigated reprogramming cells with histone deacetylase (HDAC) inhibitors, such as valproic acid. Dr. Huangfu published a paper in 2008 which showed that adding the HDAC inhibitor valproic acid improved the reprogramming efficiency in both mouse and human cells called fibroblasts. HDAC inhibitors enhance the process by preventing histones from losing acetyl groups, which in turn restores the transcription of tumor suppressors that help prevent the cell from becoming cancerous. When compared to using only Yamanaka factors, Dr. Huangfu’s initial results suggest a 100-fold increase in the reprogramming efficiency of cells using HDAC inhibitors.
Moreover, researchers are improving iPSC cell production by using new delivery systems, called vectors and supplementing Yamanaka factors with new chemical agents such as liposomes.
Stem Cell Therapy Going Mainstream
In a welcome sign, Biotech firms are bringing iPSC stem cell therapies to the clinic to treat a wide variety of diseases. For example, the journal Nature also reported last year that the Astellas Institute for Regenerative Medicine, a biotech firm in Massachusetts, has several iPSC stem cell therapies in its pipeline, including ones for macular degeneration and glaucoma. According to the firm’s chief scientific officer Robert Lanza, it takes years to work out a suitable method for making the right cell types with enough purity and in large enough quantities. As Lanza puts it,
“iPS cells are the most complex and dynamic therapies that have ever been proposed for the clinic.” Adding, “I’m the first one who wants to see these cells in the clinic, but an abundance of caution is needed.”
Autologous Stem Cell Therapy
The use of a patient’s cells, called autologous stem cell therapy is also a promising treatment approach. Stem cells which come from a patient’s body are far less likely to be rejected than those coming from someone else. Scientists hope that autologous treatments will make stem cell therapy more accurate and successful. However, these same researchers admit they do not fully understand the consequences of stem cell transplantation. Despite that fact, successes are fast outnumbering failures.
For example, in one successful experiment, scientists at the University of Pittsburgh used autologous stem cells to successfully reverse urinary incontinence in patients. To perform this novel treatment, the researchers derived stem cells from the patient’s thigh tissue and then used them to grow muscle cells, then reinjected them into the bladder.
There are several examples of failures which show that even autologous cells can carry risks. Recently, researchers injected autologous hematopoietic cells into a patient with kidney disease, and the treatment resulted in death. In another case, when scientists injected the retinas of three patients with adipose-derived autologous stem cells, it resulted in the partial or total loss of vision.
Off-the-shelf Stem Cell Therapy
Using iPSCs donated by other patients is another promising option. Technicians use these donated cells to create standardized off-the-shelf stem cell therapies Standardized cell lines dramatically cut the cost and the time of stem cell therapies by eliminating the need to analyze each patient’s cells, a costly and time-consuming process. Scientists are currently focused on creating off-the-shelf stem cell therapies.
For example, Dr. Yamanaka’s team is developing a standardized cell line to treat Parkinson’s disease. Over 10 million people worldwide suffer from Parkinson’s, and a key characteristic of the disease is the loss of cells which produce dopamine, an important neurotransmitter. Dr. Yamanaka’s team is creating off-the-shelf iPSCs to deliver fresh dopamine-producing cells to patients.
Article Takeaways
Induced pluripotent stem cells offer a potential to rejuvenate many organs and tissues. Scientists are improving upon the revolutionary Yamanaka factors. Concerns about mutations in iPSCs still linger, however, scientists are coming up with clever ways to sidestep these adverse effects. Patients have hope as biotech firms are slowly bringing iPSC stem cell therapies to the clinic.
Related Reports on Stem Cell Therapy
- Can These Revolutionary Technologies Beat Aging in Our Lifetimes? (Video)
- What everybody ought to know about stem cells (stem cell overview)
- (Part 1) Can We Reverse Stem Cell Decline and Rejuvenate Our Bodies?
- (Part 2) Can We Reverse Stem Cell Decline and Rejuvenate Our Bodies?
- Researchers grow functioning human kidney tissue (nephrons) with stem cells.
Show Us Some Love
Share this post on social media and help us spread the word– It only takes one click on any of the social media links on this page.
Follow us on social media – Google+ | Reddit
Sign up for our email list – We use your email to notify you of new articles. We will not share your email address or send you spam. You can cancel at any time.
Tell us what you think – Scroll down to enter your comments below.
References
Cover Photo: Getty Images.
Jeannine Mjoseth. “Findings: Induced pluripotent stem cells don’t increase genetic mutations.” [Press Release] National Human Genome Research Institute. Last Updated: February 8, 2017. Link to article.
Florian T. Merkle, et al. “Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations” Nature 545, 229–233 (11 May 2017) doi:10.1038/nature22312. Link to Nature article.
Stephen Chanock. “Stem cells: Subclone wars” Nature 545, 160–161 (11 May 2017) doi:10.1038/nature22490. Link to Nature article.
Huangfu, D., Maehr, R., Guo, W., Eijkelenboom, A., Snitow, M., Chen, A. E., & Melton, D. A. (2008). Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature Biotechnology, 26(7), 795-797. DOI: 10.1038/nbt1418. Link to Nature article.
Megan Scudellari. “How iPS cells changed the world.” Nature 534, 310–312 (16 June 2016) doi:10.1038/534310a. Link to Nature article.
Disclaimer
Diagnosis, Treatment, and Advice: This article is intended for educational and informational purposes only and is not a substitute for qualified, professional medical advice. The information and opinions provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. Consult a qualified and licensed physician for the diagnosis and treatment of any and all medical conditions. Experimental stem cell therapies carry a much higher risk than FDA-approved ones. Call 911, or an equivalent emergency hotline number, for all medical emergencies. As well, consult a licensed, qualified physician before changing your diet, supplement or exercise programs. Photos, Endorsements, & External Links: This article is not intended to endorse organizations, companies, or their products. Links to external websites, mention or depiction of company names or brands, are intended for illustration only and do not constitute endorsements.
After my 3rd pulmonary embolism in 2003, I was diagnosed with pulmonary fibrosis and I read that people usually die within 5 years. My specialist has me on blood thinners, prednisone and other steroids that I inhale daily.